Abstract
Background:
Hepatitis B virus (HBV) is highly endemic in many of the Pacific Island countries. Four island countries—Cook Islands, Kiribati, Niue, and Tokelau—sought to evaluate the success of their hepatitis B vaccination programs by conducting nationally representative serosurveys among children born post-vaccine introduction.
Methods:
Cook Islands, Niue, and Tokelau conducted school-based census serosurveys because of small populations. The Cook Islands tested children in second grade; Niue tested children in early childhood education through sixth grade; and Tokelau tested children in first through sixth grades. Because Kiribati has a much larger birth cohort, it conducted a one-stage stratified serosurvey among first grade students. All four countries tested children using the Alere Determine™ rapid point of care hepatitis B surface antigen (HBsAg) test.
Results:
In the three smaller countries, no children were seropositive for HBsAg (0/245 Cook Island students, 0/183 Niuean students, 0/171 Tokelau students). In Kiribati, 39 (3.3%, 95% confidence interval 2.4–4.6%) of 1249 students were HBsAg positive. Vaccination data collected in the Cook Islands and Tokelau showed high vaccination coverage in both countries with ⩾P95% birth dose coverage and 100% 3-dose coverage.
Conclusions:
The Cook Islands, Niue, and Tokelau have made remarkable progress in establishing strong vaccination programs and towards decreasing the burden of hepatitis B among children. Kiribati still needs to improve vaccination coverage to achieve the <1% HBsAg target established by the World Health Organization Western Pacific Region.
Keywords: Hepatitis B, Vaccination, Cook Islands, Niue, Tokelau, Kiribati
1. Introduction
Globally, an estimated 240 million people have chronic hepatitis B virus (HBV) infection and an estimated 600,000 die from the consequences of this infection yearly [1,2]. The burden of disease is greatest among those living in the World Health Organization (WHO) Western Pacific Region (WPR), most of whom are infected by perinatal or horizontal transmission during the first five years of life [3,4]. To help combat this public health problem, the WPR member states committed to controlling chronic HBV infection, defined as a hepatitis B surface antigen (HBsAg) seroprevalence <1% among children aged ⩾5 years, by 2017 [5].
People living in the Pacific Island Countries (PICs) have some of the highest rates of chronic HBV infection in the world, with HBsAg seroprevalence ranging from 3% to 29% in the general population [6]. Recently, four PICs—Cook Islands, Kiribati, Niue, and Tokelau—sought to evaluate their hepatitis B vaccination programs and the progress they have made towards the 2017 WPR control goal. The pre-vaccine prevalence of HBsAg in three of these countries has been described in the literature; there are no published data describing the burden in Tokelau. In the Cook Islands, two convenience sample surveys from the 1970s to 1980s showed an HBsAg seroprevalence of 4.8% and 14% [7,8]. In Kiribati, HBsAg prevalence ranged from 3.8% among children aged 12–24 months in 1998 to 27–32% among older individuals in studies done in 1985–1986 and in 1998 [9,10]. In Niue, a census survey of adults in 1980 found 11.9% HBsAg prevalence, while a survey of children in 1983 found a prevalence of 3.4% among infants and of 10.3–11% among children and adolescents aged 1–19 years [11–13]. All four countries introduced hepatitis B-containing vaccine more than 20 years ago, and they have largely achieved and maintained the Global Vaccine Action Plan target of ⩾P90% coverage with three doses of hepatitis B vaccine since 2011 (Table 1, Fig. 1). Hepatitis B vaccine birth dose (HepB-BD) coverage has been nearly universal in the Cook Islands and Niue. However, HepB-BD coverage has been below the targeted level in the two other countries, ranging from 66–84% in Kiribati and 33–100% in Tokelau since 2011 (Fig. 1).
Table 1.
Characteristics of hepatitis B control programs through vaccination in the Cook Islands, Kiribati, Niue, and Tokelau.
| Cook Islands | Kiribati | Niue | Tokelau | |
|---|---|---|---|---|
| Estimated hepatitis B surface antigen seroprevalence in the general population (%)a | 10 | 29 | 8 | Not available |
| Year hepatitis B vaccine introduced into national immunization schedule | 1989 | 1995 | 1986 | 1990 |
| Current hepatitis B vaccination schedule | <24 h, 6 weeks, 3 months, 5 months | <24 h, 6 weeks, 10 weeks, 14 weeks | <24 h, 6 weeks, 3 months, 5 months | <24 h, 6 weeks, 10 weeks, 14 weeks |
| Vaccine formulation-birth dose | Monovalent | Monovalent | Monovalent | Monovalent |
| Vaccine formulation-infant series | DTP-Hib-HepB | DTP-Hib-HepB | DTaP-Hib-HepB-IPV | DTP-Hib-HepB |
| Prenatal hepatitis B surface antigen screening | Yes | No | Yes | Yes |
| Hepatitis B immunoglobulin provided to high-risk newborns | Yes | No | Yes | Yes |
| % of deliveries assisted by a skilled birth attendant (SBA)b | 100% | 90% | 100% | 100% |
| Primary school enrollmentc | 100% | >95% | 100% | 100% |
Fig. 1.
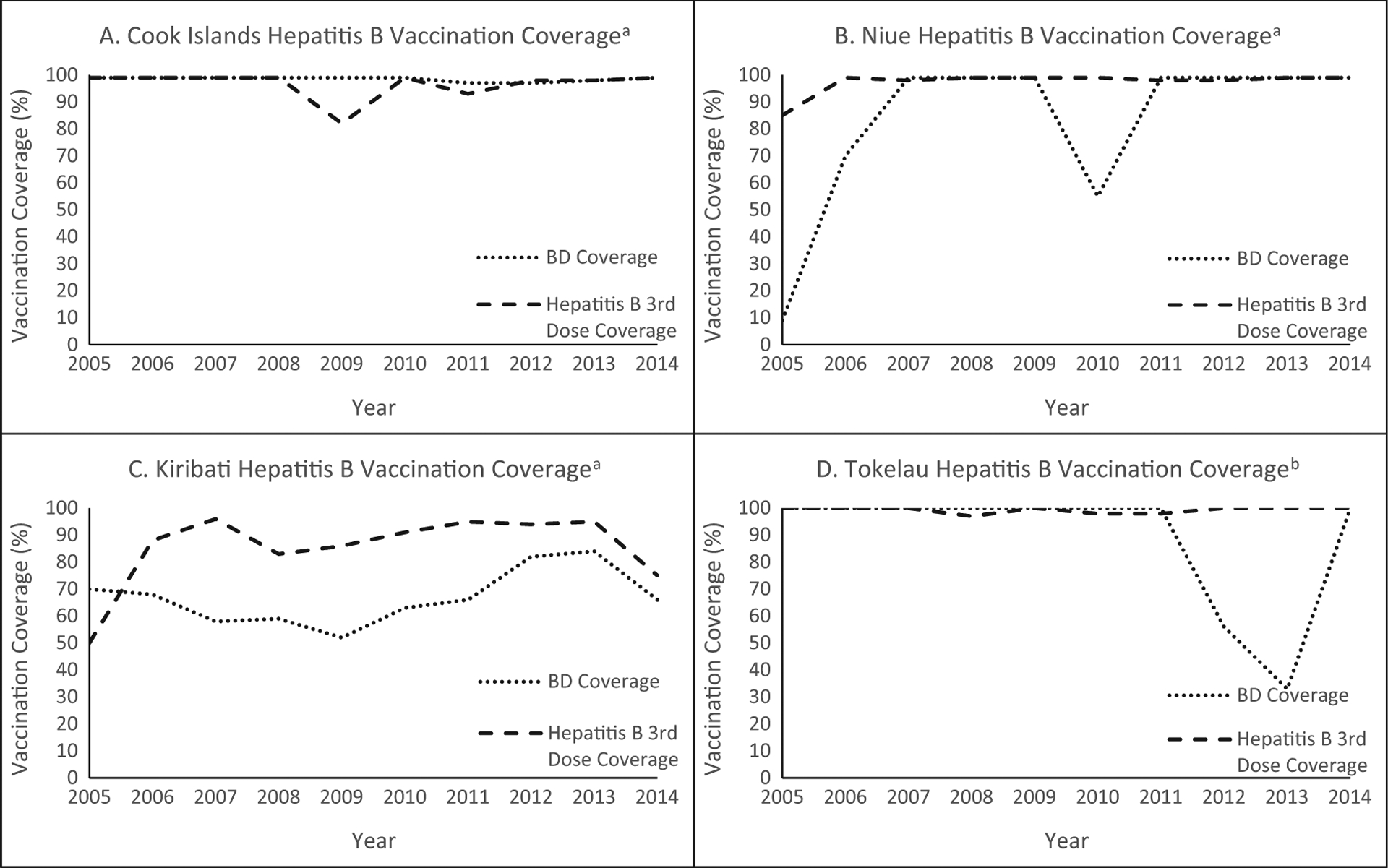
Hepatitis B birth dose (HepB-BD) and 3rd dose vaccination coverage for Cook Islands (A), Niue (B), Kiribati (C), and Tokelau (D), 2005–2014.aData Source: WHO-UNICEF Estimates [19]. bData Source: Official Country Estimates [20].
To assess the progress of the national immunization programs in reaching the 2017 WPR HBV control goal, we undertook serosurveys in the four countries to ascertain HBsAg seroprevalence among children born after the nationwide implementation of the hepatitis B vaccination program. A secondary objective of the surveys was to assess hepatitis B vaccination coverage in the studied population, if possible.
2. Methods
During 2012–2015, we conducted nationwide, cross-sectional, school-based serosurveys among children in the four countries. Primary school enrollment was estimated to be >95% in all countries.
2.1. Sample size, sampling, and study design
Table 2 summarizes the key characteristics of the hepatitis B serosurveys conducted in the four countries. In the Cook Islands, a census survey of all children in second grade was conducted. Due to logistical challenges, 12 children living on the remote islands of Penrhyn, Palmerston, and Nassau were excluded. In Niue, a census survey of all children enrolled in early childhood education through sixth grade was conducted. In Tokelau, a census survey of all children enrolled in the first through sixth grades was conducted.
Table 2.
Key characteristics of hepatitis B serosurveys conducted in Cook Islands, Kiribati, Niue, and Tokelau.
| Cook Islands, 2012 | Kiribati, 2014 | Niue, 2015 | Tokelau, 2014 | |
|---|---|---|---|---|
| Time period of survey | May-June 2012 | August 2014 | February 2015 | August-September 2014 |
| Study design | Census of grade 2 students | One stage cluster survey of grade 1 students | Census of ECEa-grade 6 students | Census of grade 1–6 students |
| Total population in selected grade(s) | 326 | 3343 | 216 | 171 |
| Exclusionary criteria | 3 remote islands (students excluded n = 12) | 2 remote islands (students excluded n = 20) | None | None |
| Number of eligible children for survey | 314 | 1293 | 216 | 171 |
ECE: Early Childhood Education.
In Kiribati, a cluster survey was conducted because of the larger number of students and access challenges. In 2014, there were 3343 first grade 1 students in Kiribati. Assuming a seroprevalence of 1% with a precision of ±0.5%, a design effect of 1.1, 2-sided 95% confidence interval (CI), α = 0.05, and accounting for the finite population, a minimum sample size of 1116 children was needed. Factoring a 15% non-response due to refusals/absentee, 1313 children were targeted for enrollment. A stratified single-stage cluster survey approach was used. To help ensure selection of some outer islands, where population sizes were smaller, two strata were defined. The inner island strata included all public and private schools in North and South Tarawa. The outer island strata included all public and private schools on all the other islands, except two schools in Kanton and Banaba Islands that are small in population (n = 20 students) and extremely difficult to access. Most schools were classified as their own primary sampling unit (PSU). However, in order to create more consistent cluster sizes, geographically close islands with small cohorts of children were clustered together to create PSUs of approximately 30–40 children; this was done for the islands of Aranuka, Maiana, Nikanau, and Onotoa. In the first stage, 13 PSUs in the inner island strata and 20 PSUs in the outer island strata were chosen by systematic random sampling, with the goal of targeting the 1313 sample size requirement. All first grade children in the selected schools were eligible for participation.
2.2. Data collection
Consent was requested from parents/caregivers prior to participation in the serosurvey. To describe possible biases that might have resulted from refusal of children to participate in the serosurvey, data were collected on key demographic characteristics and vaccination data among eligible children using a standard form irrespective of consent when possible. Vaccination data for the survey were obtained from public health vaccination records.
2.3. Specimen collection and HBsAg testing
Approximately 50 μL of blood were collected from each consented child by finger prick and tested at the school using the Alere Determine™ HBsAg point-of-care test strip (reported sensitivity: 95–100%; reported specificity: 96–100%) [14–16]. The test reports either a positive or negative result. If no control line appears, the test is considered invalid.
2.4. Data management/analysis
Data were collected using a standardized data collection form, entered into an Excel spreadsheet (Seattle, WA, USA), and analyzed using SAS v9.3 (Cary, NC, USA) and SUDAAN v10 (Research Triangle Park, NC, USA). Participants were defined as those who had consent for HBsAg testing; nonparticipants were those without consent for HBsAg testing. HepB-BD was considered to have been given within 24 h of birth if the date of administration was on the day of birth or the day after birth. Any HepB-BD was defined as a dose given before day 42 of life (first day of eligibility for a multiple-antigen vaccine) if date of administration was noted. If date of HepB-BD administration was unavailable, it was assumed that an annotation in the birth dose column of the vaccination card was valid as ‘any HepB-BD’. For the Cook Islands, Niue, and Tokelau, no confidence intervals are presented since these were census surveys. For Kiribati, estimates and Logit 95% CI for population characteristics were calculated using Taylor series variance estimation methods and accounting for the survey design, the finite population, and weights (SUDAAN v10). For Kiribati, weights were used for population characteristics, seroprevalence estimates were adjusted for non-response, and weighted proportions are presented. For this evaluation, we decided that if <25% of children in each of the countries had vaccination data for review, the results would not be representative of the population, and they are not presented.
2.5. Human subjects’ rights and ethics
Informed consent was obtained from parents/caregivers before testing. The study protocol was approved by the Ethics Review Committee at the WHO Regional Office for the Western Pacific since none of the four countries had their own ethics committee. CDC determined the activity to be human subject research in Niue, but CDC involvement did not constitute direct engagement in human subject research; therefore, it did not require CDC Institutional Review Board (IRB) review. In Kiribati, Cook Islands, and Tokelau, CDC determined the activity to be program evaluation and did not require CDC IRB review.
3. Results
3.1. Cook Islands
There were 314 eligible children in second grade (mean age 6.5 ± 0.5 years) in the Cook Islands; 45 (14%) were not present or did not have consent to participate in the serosurvey (Table 3). Nonparticipants were more likely to be non-Maori (32% of nonparticipants versus 2% of participants), and were more likely born outside of the Cook Islands (30% of nonparticipants versus 9% of participants). Among participants, 258 (96%) of 269 children had vaccination data for review. Of these 258 children, 245 (95%) received a HepB-BD ⩽24 h, 249 (97%) received any HepB-BD, and 258 (100%) received three doses of hepatitis B vaccine. Among the 45 nonparticipants, 35 (78%) had vaccination data available for review in the electronic registry. Of these 35 children, 33 (94%) received a HepB-BD ⩽24 h, 34 (97%) received any HepB-BD, and all received three doses of hepatitis B vaccine. No participants were found to be HBsAg positive.
Table 3.
Characteristics of hepatitis B serosurvey participants in the Cook Islands, Kiribati, Niue, and Tokelau.
| Cook Islands, 2012 | Kiribati, 2014 | Niue, 2015 | Tokelau, 2014 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | N | % | n | N | Weighted% | n | N | % | n | N | % | |
| Participation | 269 | 314 | 86 | 1249 | 1293 | 96 (94–99) | 183 | 216 | 85 | 171 | 171 | 100 |
| Gradea | ||||||||||||
| Early Childhood Education (ECE) | 22 | 30 | 73 | |||||||||
| 1 | 1249 | 1249 | 100 | 24 | 30 | 80 | 22 | 22 | 100 | |||
| 2 | 269 | 269 | 100 | 29 | 34 | 85 | 27 | 27 | 100 | |||
| 3 | 28 | 32 | 88 | 30 | 30 | 100 | ||||||
| 4 | 30 | 30 | 100 | 23 | 23 | 100 | ||||||
| 5 | 22 | 29 | 76 | 39 | 39 | 100 | ||||||
| 6 | 28 | 31 | 90 | 29 | 29 | 100 | ||||||
| Male | 149 | 269 | 55 | 664 | 1288 | 51 (48–54) | 98 | 171 | 57 | |||
| Born in country | 241 | 265 | 91 | 1285 | 1293 | 99 (99–100) | 59 | 171 | 35 | |||
| Ethnicity | ||||||||||||
| Maori/Polynesian | 252 | 257 | 98 | 163 | 171 | 95 | ||||||
| Caucasian | 5 | 257 | 2 | |||||||||
| Melanesian | 0 | 257 | 0 | 1 | 171 | 1 | ||||||
| Mixed | 7 | 171 | 4 | |||||||||
| Vaccination data available for review | 258 | 269 | 96 | 104 | 1293 | 9 (3–16) | 17 | 183 | 9 | 171 | 171 | 100 |
| Any Birth Dose (HepB-BD)b | 249 | 258 | 97 | 162 | 171 | 95 | ||||||
| Received HepB-BD ⩽24 h | 245 | 258 | 95 | 73 | 95 | 77 | ||||||
| Received 3+ doses of hepatitis B | 258 | 258 | 100 | 171 | 171 | 100 | ||||||
| HBsAg+ | 0 | 269 | 0 | 39 | 1249 | 3.3 (2.4–4.6) | 0 | 183 | 0 | 0 | 171 | 0 |
Tokelau: 1 child home schooled but included in survey.
Any birth dose is defined as a dose within 41 days of life (Cook Islands) or marked on vaccination card as ‘birth dose’ but no date provided (Tokelau).
3.2. Niue
There were 216 eligible children in early childhood education through sixth grade in Niue; 183 (85%) had consent to participate in the survey (Table 3). Participation rates ranged from 73–100% by grade. Information on nonparticipants was not available for review. Only 17 (9%) children, all participants, had documented age and vaccination data available for review; given the small proportion, these data were not reviewed. Of the 183 participants tested, no children were found to be HBsAg positive.
3.3. Tokelau
There were 171 eligible children in first through sixth grades, including one age-appropriate home-schooled child who was enrolled in the survey. All children had consent to participate (Table 3). The mean age of participants was 8.2 ± 1.8 years. Only 59 (35%) children were born in Tokelau; most of the rest were born in New Zealand. All children had vaccination data available for review. Of the 171 participants, 162 (95%) had documentation of receiving any HepB-BD, although date of administration was not documented for 76 children. Of the 95 participants with documented dates of vaccination, 73 (77%) received a HepB-BD ⩽24 h. Of the 59 participants born in Tokelau, all had documented dates of vaccination: 45 (76%) received a HepB-BD ⩽24 h, 52 (88%) received a HepB-BD ⩽7 days, and 55 (93%) received any HepB-BD. Of the 112 children born outside of Tokelau, 108 (96%) received a HepB-BD, though only 36 had the exact date of vaccination documented. Among these 36 children, 28 (78%) received a HepB-BD ⩽24 h, 31 (86%) received a HepB-BD ⩽7 days, and 32 (89%) received any HepB-BD. All children received three doses of hepatitis B vaccine. No children were found to be HBsAg positive.
3.4. Kiribati
There were 1293 eligible first grade children (mean age 6.4 ± 0.7 years) in the selected schools; 1253 had parental consent for participation, but four children refused on the day of the testing. Thus, 44 (4%) did not participate in the serosurvey (Table 3). Participants and nonparticipants were similar with respect to sex, current residence (inner or outer island strata) and birth in Kiribati. Vaccination data were available for review from 104 (9%) participants and from none of the nonparticipants and thus were not analyzed. Of the 1249 participants, 39 (3.3% (2.4–4.6%)) were found to be HBsAg positive. There were no seropositive children in Abemama (0/22), Aranuka (0/30), Arorae (0/19), Makin (0/40), or South Tabiteuea (0/11) islands. Seroprevalence in the other islands varied: 5.3% (4/76) in Abaiang, 10.4% (9/87) in Butaritari, 3.8% (5/131) in Kirimati, 4.5% in (1/22) Nonouti, 3.2% (2/62) in North Tabiteuea, 0.6% (1/156) in North Tarawa, 2.8% (16/576) in South Tarawa, and 5.9% (1/17) in Onotoa.
4. Discussion
The Cook Islands, Kiribati, Niue, and Tokelau have made remarkable progress towards achieving hepatitis B control through vaccination. The Cook Islands, Niue, and Tokelau had no children among the target population with evidence of chronic HBV infection, documenting that they have achieved the 2017 WPR goal of <1% chronic HBV infection rate among children at least five years old. The three countries that have achieved the goal have done so through strong HBV control programs including prenatal screening, hepatitis B immunoglobulin (HBIG) administration to high risk newborns, and high vaccination coverage with HepB-BD and subsequent doses. Despite its progress, Kiribati has not achieved the target and will need to make improvements in its vaccination program to ensure further progress is made.
The Cook Islands, Niue, and Tokelau all have reported nearly 100% 3-dose hepatitis B vaccine coverage yearly for the past ten years (Fig. 1). Vaccination data reviewed in the Cook Islands and Tokelau for their surveys corroborated the 3-dose reported coverage estimates. In addition, 95–97% of children residing in the Cook Islands and Tokelau at the time of their surveys received any HepB-BD. In contrast to reported 3-dose and any HepB-BD coverage, however, reported HepB-BD ⩽24 h coverage has sometimes fluctuated dramatically in these three small countries, since some births occurred in neighboring countries such as Australia and New Zealand. In the Cook Islands, 94–95% of both nonparticipants and participants received their HepB-BD ⩽24 h, showing that a strong perinatal prevention program exists, facilitated by the fact that there is only one maternity hospital which has a protocol for HepB-BD provision to all newborns. In Tokelau, however, only 76–78% of children born in Tokelau and 78% of children born outside of Tokelau received a HepB-BD ⩽24 h. Reasons for failing to receive a HepB-BD ⩽24 h are unknown. Even though no cases of hepatitis B were found in this survey, Tokelau needs to strengthen timely HepB-BD administration so as not to miss a prevention opportunity. In Niue, reported vaccination data cannot be corroborated in this survey since very few vaccination data were available for review. However, it is known that the reported decrease in the 2010 HepB-BD ⩽24 h coverage was due to an obstetrician not being available in country, and all women were flown to New Zealand to deliver there. These children who are born in another country might or might not receive the HepB-BD and do not get counted towards the number of doses administered in country, yet these births are counted in the denominator of eligible children. Given the small numbers of births in Niue as well as in the other islands, small changes in the numerator can have a large impact on the percentage of children vaccinated. The failure of another country to provide a HepB-BD should not be seen as a failure of the country’s vaccination program. Because of the inability to verify the vaccination coverage in the studied cohorts, Niue should conduct a chart review to ensure that timely HepB-BD is provided to every newborn.
In Kiribati, the best estimate for pre-vaccine HBsAg prevalence was 29%. The reduction in seroprevalence to 3.3% in its serosurvey, an 89% decrease, is remarkable and is the result, in large part, of the vaccination program. The failure to achieve the <1% control goal is probably due to some remaining challenges in the vaccination program, although we were unable to assess vaccination coverage in this cohort to substantiate this. Delivery of vaccination in Kiribati is challenging largely because a larger birth cohort lives on 21 inhabited islands/atolls spread over 3.4 million kilometers of ocean. However, 90% of children are reportedly born with skilled birth attendants (SBAs), and thus coverage with a HepB-BD ⩽24 h should be high, although reported coverage in 2014 was only 66% (Fig. 1). One challenge is that vaccine is not readily available for all SBAs because of insufficient cold chain for vaccine storage [17]. Kiribati has a national policy to use hepatitis B monovalent vaccine outside the cold chain, but this policy has not been implemented [17]. One priority is to ensure those SBAs working without adequate access to cold chain are trained in using hepatitis B vaccine outside the cold chain. Additionally, health facilities that have adequate cold chain should be systematically evaluated to identify barriers to administration. Birth dose is vitally important to reducing chronic HBV infection prevalence in Kiribati, where one study found 48% of mothers with chronic HBV infection had evidence of hepatitis B e antigen, indicating a greater risk for perinatal transmission [9]. Finally, the immunization program will need to strengthen routine immunization services, since reported 3-dose coverage in 2014 was only 75%.
These four serosurveys have some limitations. First, participation was only 86% in the Cook Islands and 85% in Niue. However, characteristics of nonparticipants and participants in these two countries were similar. Additionally, in the Cook Islands, vaccination coverage was similar among participants and nonparticipants, suggesting that nonparticipants probably also had minimal, if any, chronic HBV infection given the high vaccine effectiveness (>95% for 3 doses) [18]. Second, some remote locations were excluded in the Cook Islands and Kiribati. This could bias our findings towards a lower HBsAg prevalence, since children living in more remote locations might be less likely to be vaccinated than those living on closer island, but the numbers and proportion of target population children residing in those remote islands were small (4% for the Cook Islands; 0.6% for Kiribati). Third, Kiribati also has only 95% school enrollment by first grade; therefore, children out of school were not represented in its survey; these children might have less access to vaccination services and a higher burden of chronic HBV infection than children attending school. Fourth, documented vaccination data were not available for most children in Niue and Kiribati, limiting our ability to make data-driven programmatic recommendations. In Tokelau, dates of HepB-BD vaccination were not well documented, limiting statements about timeliness of receipt of HepB-BD. In Kiribati and Niue, the lack of vaccination data prevented calculation of vaccine effectives; in Niue, Tokelau, and the Cook Islands, a lack of children with chronic HBV infection prevented calculation of vaccine effectiveness, although zero cases of chronic HBV infection compared to baseline rates in the setting of high vaccination rates suggest the vaccine is highly effective. Another limitation is that we did not test children for hepatitis B antibody to core antigen making it difficult to say if children had a resolved infection. Finally, prenatal screening and administration of HBIG to high-risk newborns occurs in the Cook Islands, Niue, and Tokelau; we are unable to account for which children were born to HBsAg positive mothers and received HBIG due to lack of documentation in the vaccination records.
These four countries have made remarkable progress towards achieving control of chronic HBV infections in children. The Cook Islands, Niue, and Tokelau need to sustain their high performing vaccination programs by continuing to ensure that every child born on the island receives a HepB-BD ⩽24 h. Kiribati needs to improve both timely birth dose and 3-dose coverage in order to achieve the <1% goal. Other countries in the region that have not assessed the impact of their hepatitis B vaccination program should conduct a serosurvey to understand progress towards the goal and identify areas that need improvement.
Acknowledgements
We’d like to acknowledge the following for their support: Karen Hennessey, Eric Wiesen, and Jayaprakash Valiiakolleri; Cook Islands Director of Community Health Services Dr. Rangiau Fariu; the Tokelau Department of Health; the staffs of the three Tokelau hospitals, Lomaloma, Saint Joseph and Fenuafala; the principals and staffs of Tialeniu school in Fakaofo, Matiti School in Nukunonu, and Matauala School in Atafu; the Niue School Health Team composed of Minemaligi Pulu, Oscilyna Kulatea, Jenny Eveni, Denise Pihigia, Dr. Patricia Tatui, Dr. Waimanu Pulu and Abigail Pita; the Niue Director of Education Birtha Togahai; and the ECE/Niue Primary School teachers. We’d also like to thank the participants and their families without whom the successful completion of this work would not have been possible.
Funding
This work was supported by the World Health Organization (WHO). WHO had no direct role in this work except Akineti Bauro Nikuata who is employed by WHO and to serve as the ethics committee for the four countries.
Footnotes
Conflicts of interest
No authors have any conflicts of interest.
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012;30:2212–9. [DOI] [PubMed] [Google Scholar]
- [2].Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005;34:1329–39. [DOI] [PubMed] [Google Scholar]
- [3].Clements CJ, Baoping Y, Crouch A, Hipgrave D, Mansoor O, Nelson CB, et al. Progress in the control of hepatitis B infection in the Western Pacific Region. Vaccine 2006;24:1975–82. [DOI] [PubMed] [Google Scholar]
- [4].Hennessey K, Mendoza-Aldana J, Bayutas B, Lorenzo-Mariano KM, Diorditsa S. Hepatitis B control in the World Health Organization’s Western Pacific Region: targets, strategies, status. Vaccine 2013;31(Suppl 9):J85–92. [DOI] [PubMed] [Google Scholar]
- [5].Regional Committee of the Western Pacific. WPR/RC64.R5 Hepatitis B control through vaccination: setting the target. Manila, Philippines; 2013. <http://www.wpro.who.int/about/regional_committee/64/resolutions/WPR_RC64_R5_HepB_control.pdf?ua=1> [March 17, 20s16]. [Google Scholar]
- [6].Western Pacific Regional Plan for Hepatitis B control through immunization. Manila, Philippines; 2007. <http://www.wpro.who.int/internet/resources.ashx/EPI/docs/HepB/POA_HepB.pdf>. [Google Scholar]
- [7].Austin FJ, Maguire T, Miles JA. The occurrence of hepatitis B antigen and antibody in some population groups in the southwest Pacific region. Am J Trop Med Hyg 1974;23:489–94. [DOI] [PubMed] [Google Scholar]
- [8].Hiraiwa K, Kuroda Y, Itoh M, Katoh K. Prevalence of hepatitis B surface antigen and antibody in the Cook Islands with reference to difference in rates between islands. Fukushima J Med Sci 1987;33:109–17. [PubMed] [Google Scholar]
- [9].Wilson N, Ruff TA, Rana BJ, Leydon J, Locarnini S. The effectiveness of the infant hepatitis B immunisation program in Fiji, Kiribati, Tonga and Vanuatu. Vaccine 2000;18:3059–66. [DOI] [PubMed] [Google Scholar]
- [10].Brindle RJ, Eglin RP, Parsons AJ, Hill AV, Selkon JB. HTLV-1, HIV-1, hepatitis B and hepatitis delta in the Pacific and South-East Asia: a serological survey. Epidemiol Infect 1988;100:153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhuang H, Coulepis AG, Gust ID, Zimmet P, Taylor R, Nemaia HT. A cross sectional study of markers of hepatitis B infection in Niue. N Z Med J 1983;96:330–2. [PubMed] [Google Scholar]
- [12].Taylor R, Gust I, Nemaia HT, Whitmore J, Williamson HG, Dimitrakakis M, et al. Technical Paper No 182: Seroepidemiological studies of hepatitis B in Niue 1980–1983. Noumea, New Caledonia: South Pacific Commission; 1984. [Google Scholar]
- [13].Williamson HG, Gust ID, Dimitrakakis M, Liu SQ, Taylor R, Whitmore J, et al. Serological markers of hepatitis B infection in Niue children. N Z Med J 1985;98:275–7. [PubMed] [Google Scholar]
- [14].Lien TX, Tien NT, Chanpong GF, Cuc CT, Yen VT, Soderquist R, et al. Evaluation of rapid diagnostic tests for the detection of human immunodeficiency virus types 1 and 2, hepatitis B surface antigen, and syphilis in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg 2000;62:301–9. [DOI] [PubMed] [Google Scholar]
- [15].World Health Organization. Hepatitis B surface antigen assays: operational characteristics (Phase 1) report 1. Geneva: World Health Organization; 2001. <http://whqlibdoc.who.int/publications/2004/9241592206.pdf>. [Google Scholar]
- [16].Alere. Alere determine HBsAg package insert. Chiba, Japan. <http://www.alere.com/en/home/product-details/determine-hbsag.html> [March 17, 2016]. [Google Scholar]
- [17].Kiribati Ministry of Health. National multi-year plan for immunization in 2011–2015; 2010. <http://www.gavi.org/Country/Kiribati/Documents/CMYPs/Comprehensive-multi-year-plan-for-2011-2015/> [March 17, 2016].
- [18].World Health Organization. Hepatitis B vaccines: WHO position paper–recommendations. Vaccine 2010;28:589–90. [DOI] [PubMed] [Google Scholar]
- [19].WHO vaccine-preventable diseases: monitoring system; 2015 global summary. Geneva, Switzerland. <http://apps.who.int/immunization_monitoring/globalsummary> [March 17, 2016]. [Google Scholar]
- [20].World Health Organization Regional Office of the Western Pacific. Immunization Coverage Dashboard. <http://hiip.wpro.who.int/portal/Dashboards/Immunization/Immunizationdashboards/TabId/169/ArtMID/905/ArticleID/76/Default.aspx> [March 17, 2016].
- [21].World Health Organization Regional Office of the Western Pacific. Western Pacific Country Health Information Profiles: 2011 Revision. In: World Health Organization Western Pacific Region, editor. Geneva: World Health Orgnaization; 2011. <http://www.wpro.who.int/health_information_evidence/documents/CHIPS/en/> [March 17, 2016]. [Google Scholar]


