TO THE EDITOR:
The rapid development of safe and effective vaccines is imperative to attenuate the impact of the COVID-19 pandemic.1 Patients with multiple myeloma (MM) are at increased risk of infections, mainly because of advanced age, immunocompromised status, and concurrent comorbidities.2,3 Among nonvaccinated patients with MM and COVID-19, 77% experience moderate and severe symptoms, including the need for hospitalization.3-5 Furthermore, approximately one-third of patients with MM with COVID-19 who require hospitalization are at high risk of death.4,6,7 Recent data indicate that COVID-19 vaccination leads to a less intense humoral response in patients with MM, primarily in those without prior exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as reflected by a lower production of neutralizing antibodies (NAbs), compared with healthy controls.8,9 Active treatment with anti–B-cell maturation antigen (BCMA) regimens or anti-CD38 monoclonal antibodies that deplete B cells is considered the main negative prognostic factor for antibody production after full vaccination.9,10 Vaccines have managed to reduce severe forms of COVID-19.11-13 However, the declining humoral immunity over time and emergence of new SARS-CoV-2 variants have necessitated the administration of a booster vaccine dose.14 A third BNT162b2 dose in adults age ≥60 years was associated with significantly increased immunoglobulin G titers after 10 to 19 days, with no major adverse toxicity.15 Similar results have been reported among immunocompetent health care workers, as well as among transplant recipients.16-18
In this context, we prospectively evaluated the development of NAbs against SARS-CoV-2 in patients with MM at 30 days postvaccination with a third dose of the messenger RNA BNT162b2 vaccine (registered at www.clinicaltrials.gov as #NCT04743388). Major inclusion criteria for the participation of patients in this study included age >18 years, presence of active MM according to International Myeloma Working Group criteria,19 and eligibility for vaccination with a third dose. Major exclusion criteria included the presence of an autoimmune disorder or other active malignant disease, HIV or active hepatitis B or C infection, end-stage renal disease, and prior diagnosis of COVID-19. Dexamethasone administration was held from 2 weeks before until 1 week after each vaccine injection. No other modifications in the treatment regimens were applied. The study was approved by the Institutional Ethics Committee of General Hospital Alexandra, Athens, Greece. All patients provided written informed consent before enrollment in the study.
Serum samples were collected on the date of the booster dose (just before vaccination) and 4 weeks after. NAbs against SARS-CoV-2 were measured using a US Food and Drug Administration–approved methodology (enzyme-linked immunosorbent assay; cPass SARS-CoV-2 NAbs Detection Kit; GenScript, Piscataway, NJ).20 A NAb titer of ≥30% is considered positive, whereas a NAb titer of ≥50% has been associated with clinically relevant viral inhibition.21 All statistical analyses were performed with STATA software (version 17.0; College Station, TX). All variables were tested for normal data distribution. Nonnormally distributed data are presented as medians with interquartile ranges (IQRs). For categorical variables, the χ2 or Fisher’s exact test was used to compare the distributions for the 2 randomized groups.
The study population included 167 consecutive patients with MM (men, 58%; median age, 68 years; IQR, 60-75 years) who were vaccinated with the booster BNT162b2 dose between September and October 2021 at the same vaccination center. All patients had been fully vaccinated with the 2-dose BNT162b2, participated in the respective study for detecting NAbs after their vaccination with 2 vaccine doses (NCT04743388), had available NAb titers 1 month after their second vaccine dose (day 50 after the first dose), and received a booster BNT162b2 vaccine. At the time of vaccination, a vast majority (93.4%) of patients were receiving antimyeloma treatment. The baseline characteristics of the enrolled patients are listed in Table 1.
Table 1.
Baseline patient characteristics according to presence of NAbs >30% 1 month after 2 doses of BNT162b2 (day 50)
| Variable | <30% NAbs at day 50 (n = 57) | >30% NAbs at day 50 (n = 110) | Total (N = 167) | P |
|---|---|---|---|---|
| Age, y | 70 (66-77) | 68 (58-74) | 68 (60-75) | .03 |
| BMI, kg/m2 | 26 (24-28) | 26 (24-29) | 26 (24-29) | .47 |
| Males sex | 64.9 | 53.6 | 57.5 | .16 |
| ISS | .96 | |||
| 1 | 47.3 | 46.1 | 46.5 | |
| 2 | 27.3 | 29.4 | 28.7 | |
| 3 | 25.4 | 24.5 | 24.8 | |
| RISS | .9 | |||
| 1 | 35.9 | 32.1 | 33.3 | |
| 2 | 51.3 | 55.6 | 54.2 | |
| 3 | 12.8 | 12.4 | 12.5 | |
| Treatment category | <.001 | |||
| None | 3.5 | 8.2 | 6.6 | |
| PI based | 8.8 | 6.4 | 7.2 | |
| IMID based | 24.6 | 8.2 | 13.8 | |
| Lenalidomide maintenance | 3.5 | 19.1 | 13.8 | |
| PI plus IMID based | 8.8 | 27.2 | 21 | |
| Anti-CD38 based | 40.3 | 27.2 | 31.7 | |
| Anti-BCMA based | 10.5 | 3.6 | 6 | |
| Disease response ≥CR | 5.2 | 8.1 | 7.2 | .07 |
| Diabetes | 19.6 | 13.8 | 15.3 | .2 |
| Cardiovascular disease | 49.1 | 51.4 | 50.6 | .8 |
| Autoimmune disease | 1.8 | 2.8 | 2.4 | .8 |
| Lymphocyte count, cells per mm3 | 1200 (800-1500) | 1300 (1100-1800) | 1300 (1000-1700) | .07 |
| IgG, mg/dL | 604 (300-1145) | 965 (532-1218) | 827 (462-1213) | .01 |
| IgM, mg/dL | 19 (19-24) | 23 (19-37) | 21 (19-37) | .09 |
| IgA, mg/dL | 30 (26-125) | 93 (32-194) | 59 (28-180) | <.001 |
| NAbs at day 50, % | 20 (12-25) | 81 (59-93) | 58 (24-89) | <.001 |
| NAbs before third dose, % | 15 (7-23) | 43 (25-80) | 27 (14-66) | <.001 |
| Time from second to third dose, mo | 4.9 (4.5-5.6) | 4.9 (4.5-5.3) | 4.9 (4.5-5.4) | .4 |
Data are presented as % or median (IQR).
BMI, body mass index; CR, complete remission; Ig, immunoglobulin; IMID, immunomodulatory drug; ISS, International Staging System; PI, proteasome inhibitor; RISS, Revised International Staging System.
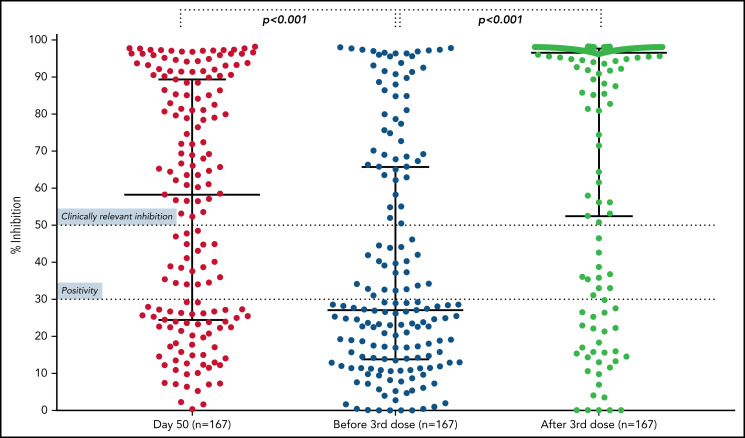
The booster dose significantly improved the humoral response in patients with MM. More specifically, the median NAb titer reached 96.7% (IQR, 52.6% to 97.8%), as compared with 27.1% (13.9% to 65.8%) before the third dose (P < .001; Figure 1; supplemental Figure 1). Overall, 114 patients (68%) had <50% NAb activity before the third dose. Among them, 75 (65.8%) experienced an increase in their NAb titer to at least 50% after the third dose. Interestingly, the patients who had achieved an NAb titer of ≥50% at 1 month after the second vaccine dose were more likely to achieve an NAb titer of ≥50% at 1 month after the third dose, as compared with those who had inferior antibody responses after the second dose (P = .001).
Figure 1.
Kinetics of NAbs in patients with MM. Nabs at at 1 month after the second dose of the BNT162b2 (median, 58.4%; IQR, 24.5% to 89.4%; day 50), at the time of the third dose (median, 27.1%; IQR, 13.9% to 65.8%; before third dose), and at 1 month after the booster dose (median, 96.7%; IQR, 52.6% to 97.8%). A significant reduction in NAbs was shown between day 50 and the time of third dose (P < .001); however, a significant increase in NAbs was evident at 1 month after the booster dose (P < .001).
Fifty-seven patients (34%) had not developed a sufficient humoral response after the second vaccination (NAb titer <30%). All of them presented with low NAb titers before the third dose (median, 14.5%; IQR, 7.2% to 23.3%). The third vaccine dose boosted the median antibody response to 38.8% (IQR, 15.6% to 92.3%; P < .001). At 1 month after the booster dose, 32 (56%) of 57 patients showed an NAb titer above the positivity threshold (≥30%), and 26 (45.6%) of 57 showed an NAb titer of ≥50%. In addition, the third dose improved the humoral response for patients with NAbs ≥30% after the second vaccine injection. Among these patients, the median NAb activity reached 97.56% (IQR, 95.6% to 97.9% vs 43%; IQR, 30.0% to 80.0% before the third dose; P < .001). Similar results were seen according to the 50% cutoff value of NAbs at 1 month after the second dose (P < .001 for all comparisons).
Furthermore, we sought to evaluate possible predictive factors for NAb response after the booster BNT162b2. In the multivariate analysis, only presence of an NAb titer ≥30% at 1 month after the second dose (odds ratio, 9.5; 95% confidence interval, 3.3-27.6) and treatment with anti-BCMA agents (odds ratio, 0.03; 95% confidence interval, 0.003-0.27) emerged as significant predictive factors for an NAb titer ≥50% at 1 month after the third dose. Interestingly, the median NAb value for patients receiving treatment with anti-BCMA therapies after the booster dose was 21.8% (IQR, 13% to 27.5%), compared with 96.8% (IQR, 61.6% to 97.8%) for patients who did not receive anti-BCMA treatment. None of the patients receiving anti-BCMA therapy achieved an NAb titer of ≥30% 1 month after the booster dose.
Our study demonstrated that a third BNT162b2 dose in patients with MM optimized the humoral response against SARS-CoV-2, as depicted by the significant increase in NAbs at 1 month after the booster dose. Importantly, ∼46% of patients with suboptimal NAb responses at 1 month after the 2-dose BNT162b2 vaccination showed NAb titers >50% at 1 month after the booster dose. These results are in accordance with the improved humoral response after a third dose of the mRNA-1273 vaccine, which induced a 49% response rate in kidney transplant recipients who did not respond after 2 vaccine doses,22 and the improved seropositivity after the third BNT162b2 dose in adults age ≥60 years.15 These responses have been associated with lower rates of confirmed infections and severe disease.
One of the main strengths of our study is the evaluation of NAbs, which have been shown to have an important predictive value for immune protection from symptomatic COVID-19.23,24 Therefore, NAb levels can be considered significant surrogates for vaccine efficacy. The main limitations of our study include the relevant limited number of patients enrolled, the absence of data on T cell–induced immune responses after booster vaccination against SARS-CoV-2, and the short follow-up period. During this time period (ie, 1 month after the booster dose), no COVID-19 cases were reported. Longer follow-up will reveal any impact of the booster vaccination on hospitalization and death resulting from COVID-19.
In conclusion, although patients with MM have an inferior humoral response against SARS-CoV-2 after vaccination with 2 doses of BNT162b2, a booster dose enhances the NAb response significantly. However, several patients do not achieve sufficient antibody response, especially those receiving treatment with anti-BCMA therapeutics. Importantly, these patients should be considered for treatment with monoclonal antibodies against SARS-CoV-2, because they are at high risk of severe COVID-19. Taking into consideration the defective immunity in patients with MM and the current COVID-19 outbreaks, self-protective measures, such as mask wearing and social distancing, remain particularly important.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This study was funded in part by SYN-ENOSIS (Piraeus, Greece) and IEMBITHEK (Athens, Greece).
Footnotes
The online version of this article contains a data supplement.
Authorship
Contribution: E.T. and M.A.D. designed research; E.T., M.G., I.N.-S., A.B., S.G., P.M., E.-D.P., M.M., N.K., E.K., and I.P.T. performed research; S.G., E.-D.P., and I.P.T. contributed vital new reagents or analytic tools; M.G., I.N.S., and A.B. analyzed data; and E.T., M.G., and I.N.S. wrote the first draft of the paper. All authors revised the final version of the manuscript and consented to submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Evangelos Terpos, Department of Clinical Therapeutics, National and Kapodistrian University of Athens, School of Medicine, Alexandra General Hospital, 80 Vas. Sofias Avenue, 11528, Athens, Greece; e-mail: eterpos@med.uoa.gr.
REFERENCES
- 1.Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, et al. Emerging treatment strategies for COVID-19 infection. Clin Exp Med. 2021;21(2):167-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumontet C, Hulin C, Dimopoulos MA, et al. A predictive model for risk of early grade ≥ 3 infection in patients with multiple myeloma not eligible for transplant: analysis of the FIRST trial. Leukemia. 2018;32(6):1404-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terpos E, Engelhardt M, Cook G, et al. Management of patients with multiple myeloma in the era of COVID-19 pandemic: a consensus paper from the European Myeloma Network (EMN). Leukemia. 2020;34(8):2000-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chari A, Samur MK, Martinez-Lopez J, et al. Clinical features associated with COVID-19 outcome in multiple myeloma: first results from the International Myeloma Society data set. Blood. 2020;136(26): 3033-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-López J, Mateos MV, Encinas C, et al. Multiple myeloma and SARS-CoV-2 infection: clinical characteristics and prognostic factors of inpatient mortality. Blood Cancer J. 2020;10(10):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelhardt M, Shoumariyeh K, Rösner A, et al. Clinical characteristics and outcome of multiple myeloma patients with concomitant COVID-19 at Comprehensive Cancer Centers in Germany. Haematologica. 2020;105(12):2872-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terpos E, Trougakos IP, Gavriatopoulou M, et al. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137(26):3674-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Oekelen O, Gleason CR, Agte S, et al. ; PVI/Seronet team . Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39(8):1028-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021;11(8):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig H, Sonneveld P, Facon T, et al. COVID-19 vaccination in patients with multiple myeloma: a consensus of the European Myeloma Network. Lancet Haematol. 2021;8(12):e934-e946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terpos E, Karalis V, Ntanasis-Stathopoulos I, et al. Robust neutralizing antibody responses 6 months post vaccination with BNT162b2: a prospective study in 308 healthy individuals. Life (Basel). 2021; 11(10):1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eliakim-Raz N, Leibovici-Weisman Y, Stemmer A, et al. Antibody titers before and after a third dose of the SARS-CoV-2 BNT162b2 vaccine in adults aged ≥60 years. JAMA. 2021;326(21):2203-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saiag E, Goldshmidt H, Sprecher E, Ben-Ami R, Bomze D. Immunogenicity of a BNT162b2 vaccine booster in health-care workers. Lancet Microbe. 2021;2(12):e650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021; 385(13):1244-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. [DOI] [PubMed] [Google Scholar]
- 20.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9): 1073-1078. [DOI] [PubMed] [Google Scholar]
- 21.Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020; 383(25):2439-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326(11):1063-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205-1211. [DOI] [PubMed] [Google Scholar]
- 24.Cromer D, Steain M, Reynaldi A, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3(1):e52-e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.