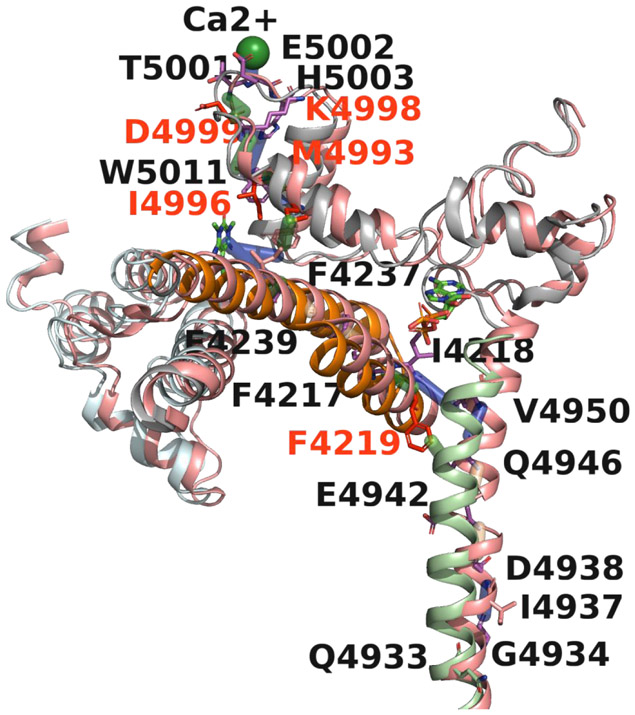
Fig 6. Structural basis of RyR1 channel gating.
Shown is a visual illustration of Ca2+ to pore communication in Ca2+/ATP/CFF bound - open and Ca2+/ATP/CFF bound- closed structures. The structure of open RyR1 is shown in salmon and closed RyR1 in color based on domains for better visualization. The pathway passing through the CFF binding site, CTD (gray), TaF (orange) and S6c (light green) domains encompassing the two allosteric interactions. Ile-4937 and Gln-4933 are the constriction sites of the closed and open channel, respectively. The differential interactions in closed-RyR1 are colored green and corresponding residues are shown in red. Twisting and outward tilting of the S6c helix from the vertical axis opened the channel to Ca2+ by rotating the Cα atoms of Ile-4937 and Gln-4933 away from the pore.