Figure 1.
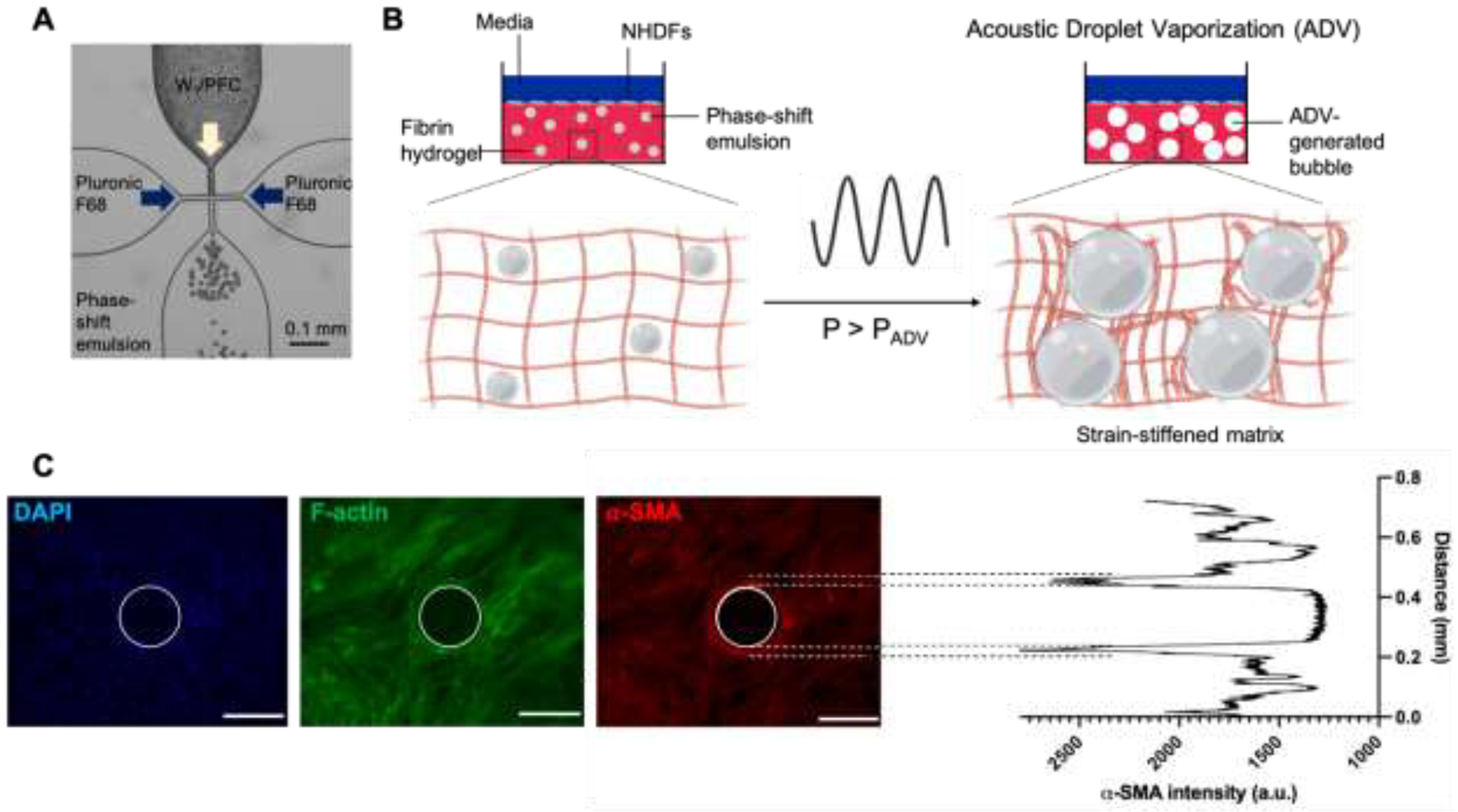
An acoustically-responsive scaffold (ARS), which consists of a phase-shift emulsion embedded within a hydrogel matrix, enables local modulation of stiffness using focused ultrasound. (A) A microfluidic chip was used to create emulsion by encapsulating a perfluoroheptane core in Pluronic F-68 surfactant. (B) The emulsion vaporizes into a gas bubble when ultrasound with a pressure exceeding the acoustic droplet vaporization threshold (i.e., P > PADV) is applied to the ARS. The generated bubble and its subsequent expansion due to in-gassing compacts the surrounding fibrin matrix, which causes stiffening. Normal human dermal fibroblasts (NHDFs) were plated on top of fibrin-based ARSs containing monodispersed emulsions either before (i.e., in situ) or after (i.e., ex situ) ultrasound exposure. (C) Representative images of NHDFs stained for nuclei (blue), F-actin (green), and α-SMA (red) on an ARS. The ultrasound-generated bubble is demarcated by a white circle (scale bars = 0.2 mm). The corresponding line plot of α-SMA intensity shows elevated signal proximal to the bubble.
