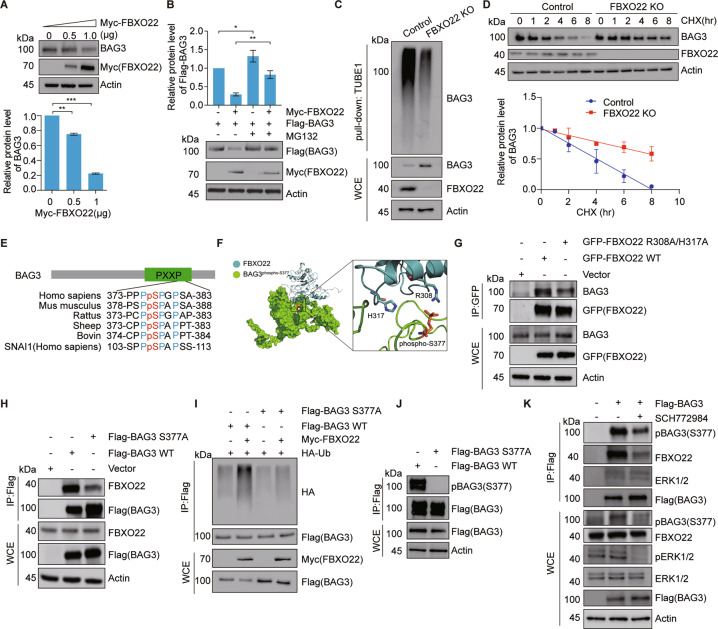
Fig. 4. FBXO22-mediated BAG3 ubiquitination and degradation requires BAG3 phosphorylation at S377.
A Immunoblot analysis of BAG3 levels after transfected with the indicated doses of Myc-FBXO22 plasmids. The quantification plot was based on scanning densitometry analysis and the relative protein levels were normalized to the control. Data are presented as mean ± SEM, n = 3 replicates. *p < 0.05, **p < 0.01, ***p < 0.001 versus control groups (Student’s t-test). B Proteasome inhibitor MG132 blocks FBXO22-induced down-regulation of BAG3. HEK 293T cells were co-transfected with Myc-FBXO22 and Flag-BAG3 for 36 h and treated with 20 μM MG132 for 5 h. Protein extracts were subjected to immunoblotting as indicated. The quantification plot was based on scanning densitometry analysis and presented as mean ± SEM, n = 3 replicates. *p < 0.05, **p < 0.01, ***p < 0.001 versus control groups (Student’s t-test). C Knockout of FBXO22 down-regulates ubiquitination of BAG3. FBXO22 KO HEK 293T cells were generated by CRISPR/Cas9 assays. Cells were treated with 20 μM MG132 for 5 h. WCE were pulled down by Tandem Ubiquitin Binding Entity 1 (TUBE1) resin [59] for ubiquitinated proteins enrichment and immunoblotted as indicated. D Knockout of FBXO22 increases the stability of BAG3. Control or FBXO22 KO cells were treated with cycloheximide for the indicated time. Protein extracts were immunoblotted for the indicated proteins. The quantification plot was based on scanning densitometry analysis and relative protein levels were normalized to the control. Data are presented as mean ± SEM, n = 3 replicates. E Alignment of amino acids corresponding to the PXXP sequence with BAG3 orthologs and another FBXO22 substrate SNAI1. F Structural model of the protein–protein interaction between FBXO22 (blue) and S377 phosphorylated BAG3 (green). Phospho-S377 was the critical phosphorylation site in the interaction. G Immunoblot analysis of the interaction between BAG3 and the FBXO22 mutant. HEK 293 T cells were transfected with GFP-FBXO22 or GFP-FBXO22-R308A/H317A mutated plasmids for 36 h. WCE were immunoprecipitated by anti-GFP beads and immunoblotted as indicated. H BAG3 S377 mutation attenuates the interaction between FBXO22 and BAG3. HEK 293 T cells were transfected with Flag-BAG3 WT or Flag-BAG3 S377A mutant for 36 h. WCE were immunoprecipitated by anti-Flag M2 resin and immunoblotted as indicated. I BAG3 S377 mutation decreases ubiquitination of BAG3. HEK 293T cells were co-transfected with Myc-FBXO22, HA-ubiquitin, and Flag-BAG3 WT or Flag-BAG3 S377A mutant for 36 h. WCE were immunoprecipitated by anti-Flag M2 resin and immunoblotted as indicated. J Antibody specificity detection of anti-phospho-BAG3 (S377) antibody. HEK 293T cells were transfected with Flag-BAG3 WT or Flag-BAG3 S377A mutant for 36 h. WCE were immunoprecipitated by anti-Flag M2 resin and immunoblotted as indicated. K ERK inhibition decreases phosphorylation of BAG3 S377, increases protein levels of BAG3, and attenuates the interaction of BAG3 and FBXO22. HEK 293T cells were transfected with EV or Flag-BAG3 for 24 h and treated with ERK1/2 inhibitor SCH772984. WCE were immunoprecipitated by anti-Flag M2 resin and immunoblotted as indicated.