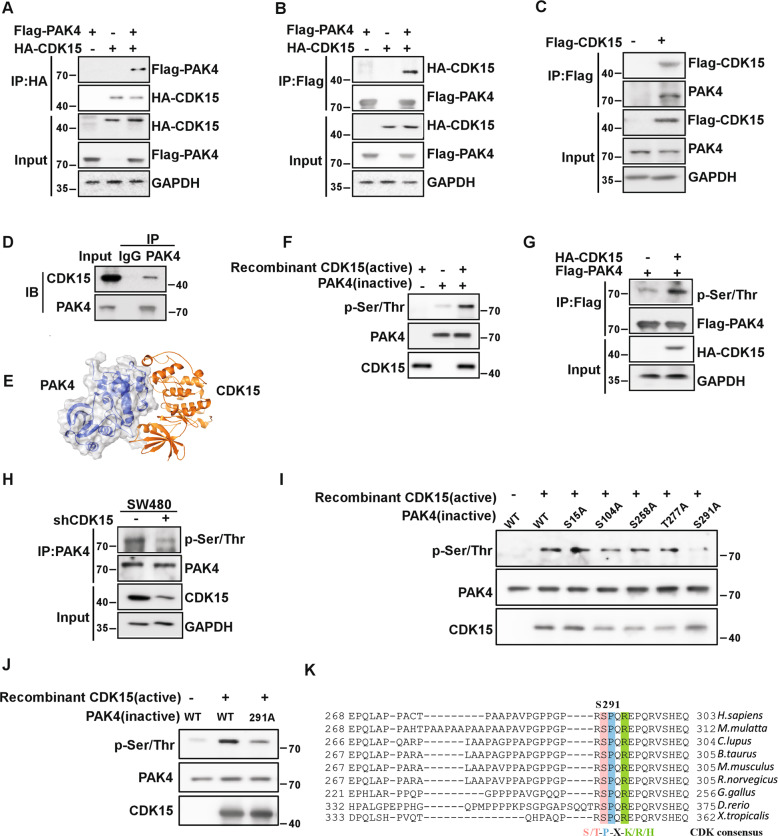
Fig. 4. CDK15 binds and phosphorylates PAK4 in vitro.
A, B CDK15 and PAK4 bind with each other exogenously. Flag-PAK4, HA-CDK15, and mock plasmids were co-transfected into HEK293T cells. At 48 h after transfection, proteins were extracted. The FLAG- or HA-tagged proteins were immunoprecipitated with anti-HA or anti-Flag, and western blot was performed. C, D CDK15 and PAK4 bind with each other endogenously. HA-CDK15 and mock plasmids were co-transfected into HCT116 cells as indicated in C. At 48 h after transfection, proteins were extracted and immunoprecipitated with anti-HA. Endogenous PAK4 was detected by western blot in C. Proteins extracted from SW480 were immunoprecipitated by anti-PAK4, and immunoprecipitated complexes were detected using anti-CDK15, in D. E Modeling of CDK15 binding with PAK4. CDK15 is colored yellow, and PAK4 is colored blue with 70% transparency. F Active CDK15 was incubated with recombinant PAK4 protein in kinase reaction buffer for 30 min at 30 °C and stopped by SDS sample buffer. Phosphorylation signals were detected by western blot. Active CDK15 was purified from HEK293T cells, while recombinant PAK4 protein was purified from BL-21. G HEK293T cells were co-transfected with the indicated constructs. Cell lysates were immunoprecipitated with anti-Flag and immunoblotted with anti-p-Ser/Thr. H SW480 cells with stable knockdown of CDK15 were immunoprecipitated with anti-PAK4, and the immunoprecipitated complex was detected by anti-p-Ser/Thr in western blot. I Active CDK15 was incubated with the indicated PAK4 mutated protein in kinase reaction buffer. Phosphorylation signals were detected by western blot. J Active CDK15 incubated with PAK4 protein harboring S291A mutation in kinase reaction buffer and then were subjected to western blot. K Sequence alignment of CDK15 phosphorylation consensus within PAK4 orthologs among different species. Phosphorylated serine residue is highlighted in red, proline at the n + 1 position is highlighted in blue, and arginine at the n + 3 position is highlighted in green.