Abstract
Background:
Resistance to endocrine therapy has been a major obstacle in the management of hormone receptor (HR)-positive metastatic breast cancer (MBC). Meanwhile, a number of treatments are available to such patients, and physicians often encounter difficulties in choosing the most appropriate treatments for individual patients. The combination of CDK 4/6 inhibitors (CDKi) and endocrine therapy has now become a standard treatment for HR-positive and human epidermal growth factor receptor 2 (HER2)-negative MBC. However, no predictive markers for CDKi-based treatments have been established. Considering their side effects and the financial burden on patients, identifying such markers is crucial.
Methods:
Clinicopathological features of 107 patients with HR-positive HER2-negative MBC, who received CDKi-based treatments at our institution were retrospectively investigated. HR status in distant metastatic lesions and immunocompetent cells in peripheral blood were also studied.
Results:
Progression-free survival (PFS) was significantly shorter in patients whose primary tumour was high grade (P = 0.016) or high neutrophil-to-lymphocyte ratio (NLR) at baseline (P = 0.017). Meanwhile, there were no differences in other factors, such as expression levels of hormone receptors. Patients whose metastatic lesions were of low tumour grade or high Ki67 labelling index had longer PFS, and such trends were more obvious than primary lesions.
Conclusion:
Our data indicate that tumour grade in primary lesion and NLR are potential predictive factors for CDKi-based treatments. Moreover, pathological assessment of metastatic lesions might also be useful.
Keywords: Breast cancer, CDK 4/6 inhibitor, predictive marker, neutrophil-to-lymphocyte ratio, metastatic lesion
Introduction
CDK 4/6 inhibitor introduction to hormone receptor-positive metastatic breast cancer
Resistance to endocrine therapy has been a major hurdle in the management of hormone receptor (HR)-positive metastatic breast cancer (MBC). Cyclin-dependent kinase (CDK) 4/6 inhibitors (CDKi) are oral drugs that decrease retinoblastoma protein phosphorylation and block cell cycle progression. In randomized studies, combinations of CDKi and endocrine therapy showed definitive clinical benefit, with significantly longer progression-free survival (PFS) compared with endocrine therapy alone.1-4 Following these results, CDKi has now become a standard treatment for HR-positive and human epidermal growth factor receptor 2 (HER2)-negative MBC.
Current problems of CDKi
MBC is generally considered to be incurable. For patients with HR-positive MBC, a number of treatment options including endocrine and chemotherapies are available, but there is no absolute rule for choosing the optimal drug. Drugs are selected based on MBC conditions, such as patient age and organs with metastases. CDKi showed its clinical benefit when administered in early lines of treatment,1-4 but it is not always easy to select CDKi-based treatments considering their side effects and financial burden on patients. Thus, physicians often encounter difficulties in choosing the most appropriate treatments for individual patients because individual MBCs respond differently, even to the same treatments. Therefore, identifying predictive factors for therapeutic efficacy of CDKi is crucial.
Several studies have investigated predictive markers of CDKi response.5-8 Phosphorylation of CDK4 at T172 might predict sensitivity to Palbociclib. 9 mRNA expression levels of cyclin E1, a CDK regulator in the G1/S phase transition of the cell cycle, were high in primary tumours of responders to palbociclib. 5 Inhibition of MDM2, a regulator of p53, was found to boost the effect of CDKi both in vitro and vivo. 10 PIK3CA in circulating tumour DNA (ctDNA) was also tested in another study. 6 Early reduction of PIK3CA in circulating tumour ctDNA was observed in patients who showed good response to palbociclib-based treatments. Aberrant FGFR (fibroblast growth factor receptor) signalling is reported to mediate resistance to CDKi and FGFR1/2 amplification in ctDNA was associated with shorter progression-free survival in patients treated with ribociclib. 11 However, evidence for markers predicting treatment effects of CDKi remains insufficient.
Herein, we retrospectively investigated clinicopathological features to identify predictive factors of CDKi-based treatments in patients with HR-positive, HER2-negative MBC, including HR status in distant metastatic lesions and immunocompetent cells in peripheral blood.
Patients and Methods
Patients and treatments
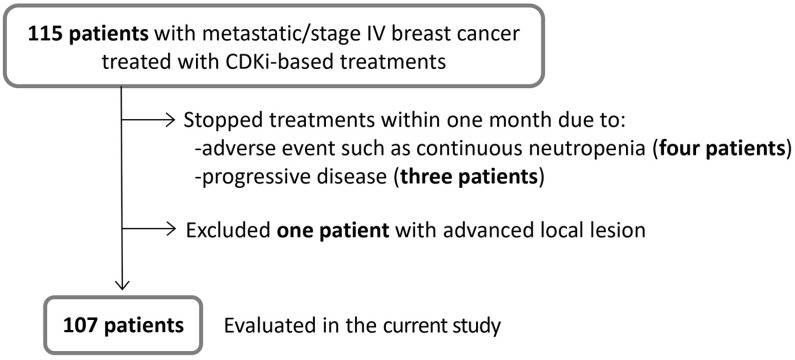
We investigated 115 patients with metastatic/stage IV breast cancer who had started CDKi-based treatments at our department during the period from December 2017 to December 2019. The selection criteria are shown in Figure 1. We excluded seven patients from the current study who had CDKi-based treatment for less than 1 month. Details of the seven excluded patients are; four discontinued treatment due to adverse events, such as continuous bone marrow suppression, and eventually switched to other treatments, three developed rapid progression of disease and were transferred to palliative care. We also excluded one patient with advanced local lesion, thus eight patients in total were excluded from this study.
Figure 1.
Flowchart of eligible patients. Patient selection criteria for the current study are shown. Among 115 patients who received CDKi-based treatment at our department, eight were excluded for the reasons indicated. In total, we included 107 patients.
Clinicopathological features of the 107 patients are shown in Table 1. Mean patient age at the time of starting CDKi-based treatments was 58 (range: 35-87) years. All primary tumours were oestrogen receptor (ER)-positive and HER2-negative. Eighty-six patients developed metastatic disease after undergoing curative surgery for primary breast cancer, while 21 had stage IV disease. Meanwhile, the mean disease-free interval (DFI) after curative surgery for the 86 patients who developed metastasis after surgery was 73 (range: 7-321) months. Visceral metastases, defined as the soft internal organs, such as the lungs and digestive organs, developed in 67 patients (63%). CDKi was administered as the first, second, third, or fourth or more line of endocrine therapy for metastatic disease in 30%, 26%, 17% and 27% of patients, respectively. Palbociclib and abemaciclib were administered in 87 (81%) and 20 patients (19%) at the standard dose of 125 mg/day and 300 mg/day, respectively, in combination with endocrine therapy, but drug doses were reduced as needed from the start of or during treatment, such as in cases with neutropenia. To date, only these two CDKi have been approved for MBC in our country.
Table 1.
Clinicopathological features of the 107 patients.
| Variables | n, (range/%) | ||
|---|---|---|---|
| Age (mean) a | 58 (35-87) | ||
| Characteristics of primary tumour | Histology | IBC-NST | 88 (82%) |
| Others | 14 (13%) | ||
| Unknown | 5 (5%) | ||
| Tumour grade | High | 9 (8%) | |
| Intermediate/low | 73 (68%) | ||
| Unknown | 25 (23%) | ||
| Ki67 L.I. (mean) | 33% (2-85) | ||
| ER | High | 83 (78%) | |
| Low | 13 (12%) | ||
| Unknown | 11 (10%) | ||
| PgR | High | 39 (36%) | |
| Low | 59 (55%) | ||
| Unknown | 9 (8%) | ||
| Stage IV | Yes | 21 (20%) | |
| No | 86 (80%) | ||
| DFI (mean, months) b | 73 (7-321) | ||
| Visceral metastasis | Yes | 67 (63%) | |
| No | 40 (37%) | ||
| Organs of MBC | Bone | 67 (63%) | |
| Liver | 27 (25%) | ||
| Lungs | 38 (36%) | ||
| Brain | 2 (2%) | ||
| Others | 51 (48%) | ||
| Number of previous treatments c | ET | 0/1 | 60 (56%) |
| ⩾2 | 47 (44%) | ||
| Chemotherapy | 0/1 | 81 (76%) | |
| ⩾2 | 26 (24%) | ||
| CDKi | Palbociclib | 87 (81%) | |
| Abemaciclib | 20 (19%) | ||
| Combined ET with CDKi | Fulvestrant | 64 (60%) | |
| Aromatase inhibitors | 43 (40%) | ||
| Immune cells in peripheral blood d | ALC (mean, per μL) | 1505 (235-3079) | |
| NLR (mean) | 2.5 (0.6-12.7) | ||
| PLR (mean) | 185 (67-608) |
Abbreviations: ALC, absolute lymphocyte count; DFI, disease-free interval; ET, endocrine therapy; IBC, invasive breast carcinoma; L.I., labelling index; MBC, metastatic breast cancer; NLR, neutrophil-to-lymphocyte ratio; NST, no special type; PLR, platelet-to-lymphocyte ratio.
At the time of starting CDKi treatment.
For patients who underwent curative surgery for the primary tumour.
For MBC.
At the time of the first administration of CDKi.
This study was carried out with approval from the ethics committee of our hospital (no: 16-096). Informed consent from participants were obtained in an opt-out manner. The research plan is presented on the homepage of our hospital and all patients were offered the choice to opt-out of the study at any time.
Evaluation of progression-free survival
Progression-free survival (PFS) was defined as the period from CDKi initiation to the time when disease was clinically judged to have progressed. Since this study was conducted retrospectively, the timing of evaluation, determined by individual clinicians based on the disease state, differed among patients.
Pathological diagnosis
Pathological examinations for primary tumours were carried out at our hospital by two experienced pathologists. Histological type was judged based on the WHO Classification of Tumours of the Breast (5th Edition). Tumour grade was evaluated based on the modified Bloom-Richardson histologic grading system. 12 Regarding Ki67 labelling index (L.I.), a hot spot was chosen, and cells positive for nuclear Ki67 were then semi-quantitatively counted under high-power field. ER and progesterone receptor (PgR) statuses were assessed semi-quantitatively and reported as positive when 1% or more of the nuclei of cancer cells showed staining. Furthermore, we categorized ER and PgR expression into high and low based on the proportion of positive stained cells. We employed 66% as cut-off value for the grouping in the current study, ie, a tumour with ER/PgR expression over 66% of cells was defined as ‘high’, considering that some primary tumours were assessed with Allred scoring at previous hospitals 13 and surgical specimens were not available. Yet, ER expression levels in 11 cases (10%) were unable to be assessed, but appeared positive based on clinical records. HER2 was judged to be positive if more than 10% of tumour cells showed strong staining of the entire cell membrane, or HER2/neu gene amplification was confirmed by fluorescence in situ hybridization. HER2-positive cases were excluded from this study.
Among the 107 cases, metastatic sites were biopsied in 35 cases in which patients developed MBC. Tumour grade, Ki67 L.I., ER, and PgR in these biopsy specimens were also examined in relation to PFS. Metastatic sites of biopsy in these 35 cases were: bone in five (14%), liver in three (9%), lungs in five (14%), pleural in three (9%), gastrointestinal tract in two (6%) and others, such as soft tissue, in 17 cases (49%).
Evaluation of immune cells in peripheral blood
The absolute lymphocyte count (ALC), the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) were calculated from the baseline laboratory data obtained on the day of the first CDKi administration. Haematological analysis was performed with an XE-5000 (Sysmex Corporation, Japan) according to the flow cytometry method for measuring and differentiating cell types in whole blood.
For the evaluation of factors related to PFS, we employed mean values for each parameter as the cut-off value for distinguishing between high and low groups, obtaining values of 1505 μL for ALC, 2.5 for NLR, and 185 for PLR (Table 1), as no absolute cut-off values have been established for these markers.
Statistical analysis
Statistical analyses were performed using JMP 11.2.1 statistical software (SAS Institute Inc., Cary, NC, USA). For analysis of PFS, a Cox proportional hazard model was applied to evaluate prognostic effects of the variables with a 95% confidence interval. For the full-model analysis, we selected variables based on their clinical significance. Age, tumour grade, expression level of ER in primary tumour, DFI, presence of visceral metastasis, number of previous endocrine therapies for MBC, ALC and NLR were thus chosen. Kaplan-Meier curves were estimated, and the log-rank test was used for comparisons of the survival distributions of the two patient groups. A P < 0.05 was considered to indicate a statistically significant difference.
Results
Clinicopathological features related with PFS
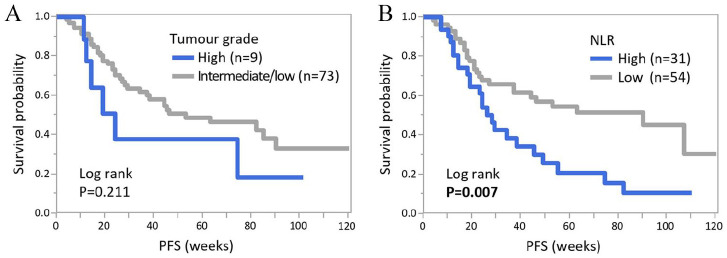
Mean PFS was 43 weeks (range: 4-120). With the Cox hazard model, the relationships between clinicopathological features and PFS were investigated (Table 2). As to the cut-off values for continuous variables (eg, age and DFI), mean values were employed as indicated in the Methods section and Table 1. Among the factors investigated, tumour grade and Ki67 L.I. of the metastatic lesion, presence of visceral metastasis, the number of previous treatments for MBC, ALC, NLR and PLR were associated with PFS in the univariate analysis. Meanwhile, PFS did not differ in other variables, such as age, HR status of the primary tumours and combined endocrine therapy with CDKi. By multivariate analysis, tumour grade of the primary lesion and NLR remained independent predictive factors for the length of PFS (P = 0.016 and 0.017, respectively). Patients with high-grade tumours or high NLR at baseline had significantly shorter PFS. Next, we created Kaplan-Meier curves of PFS for tumour grade and NLR (Figure 2). With the log-rank test, NLR showed a significant difference in PFS (P = 0.007), consistent with the aforementioned results of the univariate analysis.
Table 2.
The relationships between PFS and clinicopathological features in the 107 patients.
| Variables | Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age a | ⩾58 vs <58 | 1.45 | 0.85-2.47 | 0.175 | 1.80 | 0.66-4.89 | 0.248 |
| Primary lesion (n = 107) | |||||||
| Histology | IBC-NST vs others | 1.38 | 0.67-2.84 | 0.380 | |||
| Tumour grade | High vs intermediate/low | 0.58 | 0.24-1.38 | 0.215 | 0.21 | 0.06-0.74 | 0.016 |
| Ki67 L.I. | ⩾33% vs <33% | 1.08 | 0.54-2.17 | 0.831 | |||
| ER | High vs low | 1.03 | 0.41-2.60 | 0.950 | 1.66 | 0.45-6.18 | 0.450 |
| PgR | High vs low/negative | 1.01 | 0.58-1.74 | 0.979 | |||
| Metastatic lesion (n = 35) | |||||||
| Tumour grade | High vs intermediate/low | 0.15 | 0.05-0.44 | < 0.001 | |||
| Ki67 L.I. | ⩾33% vs <33% | 0.32 | 0.11-0.94 | 0.038 | |||
| ER | High vs low/negative | 2.01 | 0.78-5.17 | 0.146 | |||
| PgR | High vs low/negative | 2.49 | 0.80-7.80 | 0.117 | |||
| DFI (months) b | ⩾73 vs <73 | 1.03 | 0.57-1.83 | 0.933 | 1.52 | 0.52-4.48 | 0.445 |
| Visceral metastasis | Yes vs no | 0.51 | 0.29-0.90 | 0.021 | 0.61 | 0.26-1.45 | 0.261 |
| Number of previous treatments for MBC | |||||||
| ET | 0/1 vs ⩾ 2 | 2.29 | 1.35-3.88 | 0.002 | 2.42 | 0.95-6.18 | 0.065 |
| Chemotherapy | 0/1 vs ⩾ 2 | 1.95 | 1.13-3.35 | 0.016 | |||
| Combined ET | Ful vs aromatase inhibitors | 0.92 | 0.54-1.54 | 0.748 | |||
| ALC (per μL) c | ⩾1505 vs <1505 | 2.13 | 1.19-3.82 | 0.011 | 1.33 | 0.52-3.38 | 0.551 |
| NLR c | ⩾2.5 vs <2.5 | 0.47 | 0.27-0.82 | 0.008 | 0.31 | 0.12-0.81 | 0.017 |
| PLR c | ⩾ 185 vs < 185 | 0.46 | 0.26-0.81 | 0.007 | |||
Abbreviations: ALC, absolute lymphocyte count; CI, confidence interval; DFI, disease-free interval; ET, endocrine therapy; Ful, fulvestrant; HR, hazard ratio;BC, invasive breast carcinoma; L.I., labelling index; MBC, metastatic breast cancer; NLR, neutrophil-to-lymphocyte ratio; NST, no special type;LR, platelet-to-lymphocyte ratio.
At the time of starting CDKi treatment.
For patients who underwent curative surgery for the primary tumour.
At the time of the first administration of CDKi.
Figure 2.
Kaplan-Meier curves of PFS according to tumour grade and NLR. Kaplan-Meier curves of PFS according to tumour grade (A) and NLR (B) are shown. NLR indicates neutrophil-to-lymphocyte ratio; PFS, Progression-free survival.
The liver might be a potent indicator among visceral organ metastasis sites
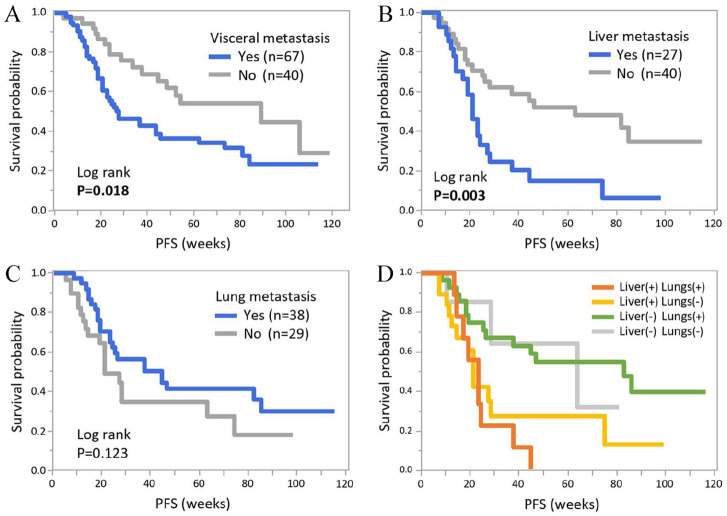
Presence of visceral metastasis was related to shorter PFS in the aforementioned univariate analysis and corresponding results were confirmed with Kaplan-Meier curves of PFS (Figure 3A, P = 0.018). Because metastatic sites are frequently key factors when treatment selections are made in clinical practice, we further analysed PFS according to the status of visceral metastasis. Among the 67 patients with visceral metastasis, patients with liver metastasis had significantly shorter PFS than those without it (P = 0.003), while there was no difference in PFS relating to lung metastasis (P = 0.123, Figure 3B and C). The presence of liver metastasis seemed to be crucial, with the shortest PFS among patients with visceral metastases (Figure 3D).
Figure 3.
Kaplan-Meier curves of PFS according to status of visceral metastasis. Kaplan-Meier curves of PFS according to visceral metastatic status are shown. (A) Comparison of PFS with/without visceral metastasis. Among 67 patients with visceral metastasis, Kaplan-Meier curves according to presence of liver (B) and lung metastases (C) are shown. (D) Comparisons between patients with metastasis in the liver and lungs. PFS indicates Progression-free survival.
Pathological assessment of metastatic lesions might be superior to primary breast cancer in terms of predicting response to CDKi
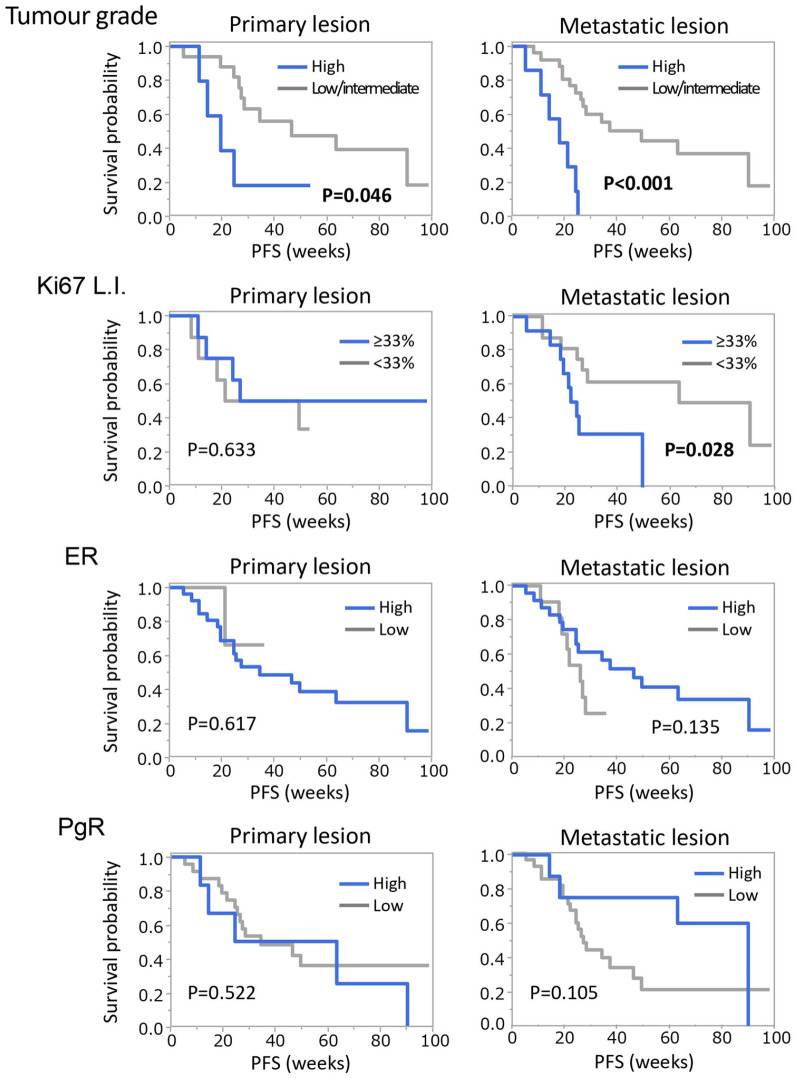
In the current study, we conducted pathological assessment on metastatic lesions (n = 35). In univariate analysis (shown in Table 2), tumour grade and Ki67 L.I. were related with the length of PFS, indicating that patients with low malignant metastatic tumours respond well to CDKi. Tumours with high expression of HR also seemed to have a similar trend towards the treatments, although the differences were not statistically significant. Hence, we further analysed these data from the 35 patients. Table 3 shows comparisons of pathological assessment between primary and metastatic lesions. Between primary and metastatic lesions, only ER showed a significant difference: ER-high rate dropped from 90% to 69% during the time course (P = 0.042). Meanwhile, other factors did not markedly change. Finally, Kaplan-Meier curves of PFS according to these variables were also drawn (Figure 4). Compared to primary lesions, metastatic lesions showed better separation in PFS, although a statistically significant difference was observed only in tumour grade (P < 0.001) and Ki67 L.I. (P = 0.028).
Table 3.
Pathological features of primary and metastatic lesions (n = 35).
| Variables | Primary tumour | Metastatic lesion | p value | |
|---|---|---|---|---|
| Tumour grade | High | 5 | 7 | 0.894 |
| Intermediate/low | 17 | 26 | ||
| Unknown | 13 | 2 | ||
| Ki67 L.I. (mean) | 41.5% | 42.1% | 0.950 | |
| ER | High | 26 | 24 | 0.042 |
| Low/negative | 3 | 11 | ||
| Unknown | 6* | 0 | ||
| PgR | High | 6 | 8 | 0.780 |
| Low/negative | 24 | 27 | ||
| Unknown | 5** | 0 |
Abbreviation: L.I., labelling index.
All six cases were ER-positive but unable to be assessed for semi-quantitative analysis.
includes three cases positive for PgR but unable to be assessed for semi-quantitative analysis.
Figure 4.
Comparisons of PFS according to pathological assessment between primary and metastatic lesions. Kaplan-Meier curves of PFS according to tumour grade, Ki67 L.I., ER and PgR in primary and metastatic lesions of 35 patients, who underwent biopsy of metastatic lesions, are shown. PFS indicates Progression-free survival.
Discussion
We revealed that tumour grade of primary lesion and NLR were independent factors related to the length of PFS. Patients with high-grade primary tumours or high NLR at baseline had significantly shorter PFS. Meanwhile, there was no statistically significant difference in the number of previous ET in multivariate analysis. Some evidence from large prospective clinical studies suggest that early administration of CDKi will be more beneficial,1-3 but our data suggest the benefit of CDKi for MBC patients, even when administered late in clinical practice.
As to immune cells in peripheral blood, patients with high NLR had significantly shorter PFS. NLR is an established prognostic marker for breast cancer patients, and high NLR is associated with poor outcomes.14,15 High NLR, indicating a degree of inflammation, might exert a negative impact on the host immune system, including the activities of lymphocytes and, consequently, allow tumour progression.16,17 However, the potential of NLR as a predictive marker is relatively unstudied. Some reports indicate that a low NLR correlates with responsiveness to neo-adjuvant chemotherapy.18,19 Myojin et al 20 reported that high NLR at baseline was independently related to poor response to eribulin-based treatments in MBC. Odan et al 8 recently reported that high NLR was associated with shorter time-to-treatment discontinuation in palbociclib-based treatments and our data were consistent with their results. Yet, underlying mechanisms are still largely unknown, and we believe that it merits further investigation with additional clinical data.
Assessing biomarkers on biopsy specimen of metastases is recommended, if clinically feasible, by international consensus guidelines for advanced breast cancer by the European School of Oncology (ESO) and the European Society for Medical Oncology (ESMO). 21 However, depending on the organ affected, biopsy of a metastatic lesion can be invasive, thus there are safety issues. It is always the physician’s responsibility to consider the value of biopsy for the patient (ie, whether it is necessary for decision-making). Technically, CT-guided lung and liver biopsies, for example, can be performed only at hospitals with experienced physicians. Consequently, the level of available medical care might vary across different regions and countries. Moreover, tumour heterogeneity among metastases might cause a problem when metastases develop in multiple organs. For instance, the expression of PD-L1 was lower in liver metastases than in other organs. 22 It is important to recognize such problems to be overcome in the future. In the current study, biopsy specimens from metastatic lesions showed a trend of longer PFS in patients with HR-high MBC. Moreover, tumours with low grade and Ki67 L.I., ie, biologically low malignant tumours, had clearly longer PFS. It is easy to imagine that tumour characteristics sometimes change during time course. Interestingly, only ER levels changed in our cohort. The ER-high rate significantly dropped from primary to metastatic lesions but tumour grade and other markers did not change. The discordance of biomarkers such as ER between primary and metastatic lesions, observed at a certain rate, has been well recognized in a number of studies.23-26 While there is no consensus on how to interpret discordant results, 21 our data indicates that the expression of biomarkers in metastases might more precisely predict the therapeutic effect of CDKi than in primary tumours. Although sample numbers were relatively small, our data indicate that pathological assessment of metastatic lesions might be potent for predicting treatment effect. Additionally, liquid biopsy is a potentially useful tool for developing predictive markers. Some studies employing ctDNA examination have reported positive results as mentioned above.6,11 We believe that circulating tumour cells (CTCs) should also be employed, which can be subjected to different immunostaining. 27
Patients with visceral metastases can expect a larger benefit from adding CDKi to endocrine therapy.1,2,28 However, such patients reportedly have shorter PFS than those who do not have visceral metastasis,29-31 and our results were consistent with these previous reports. Direct comparisons between visceral organs were difficult because some patients had multiple sites of metastases, but our data clearly showed that patients with liver metastasis had significantly shorter PFS than those without it, while there was no difference in PFS relating to lung metastasis. Therefore, our data suggest that treatment selection for patients with liver metastasis should be more carefully assessed when CDKi therapy is considered. In other words, chemotherapy may be prioritized in some such cases.
The main limitations of this study were the retrospective design and the lack of a control group treated with other drugs. Comparisons with other drugs are crucial for giving better treatment options. Treatment responses based on imaging findings should also be evaluated, not only PFS. Another limitation is that the current analysis was conducted in a small population at a single institution. A large-scale validation study with a randomized population, regardless of region or country, is warranted. We hope that such studies will be performed in the near future.
In conclusion, we revealed that tumour grade in primary lesion and NLR were independent factors related to the length of PFS. Moreover, our data raise the possibility of the usefulness of pathological assessment of metastatic lesions for predicting the treatment effect of CDKi.
Acknowledgments
We sincerely appreciate Clear Science Pty Ltd for language editing.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: Conception and design: Y.H., T.U., and K.N.; Development of methodology: A.S., Y.H., and Y.I.; Acquisition of data: A.S., Y.H., Y.I., T.U., K.N., and A.A.; Analysis and interpretation of data (eg, statistical analysis): Y.H.; Writing, review, and/or revision of the: A.S., Y.H., Y.I., T.U., and K.N.; Study supervision: MS.
Availability of Data and Materials: All data generated or analysed during this study are included in this published article.
Ethics Approval and Consent to Participate: This study was carried out with approval from the ethics committee of our hospital (no: 16-096). All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent from participants were obtained in an opt-out manner. The research plan is presented on the homepage of our hospital and all patients were offered the choice to opt-out of the study at any time.
ORCID iD: Yoshiya Horimoto  https://orcid.org/0000-0001-8935-0768
https://orcid.org/0000-0001-8935-0768
References
- 1. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med. 2015;373:209-219. [DOI] [PubMed] [Google Scholar]
- 2. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925-1936. [DOI] [PubMed] [Google Scholar]
- 3. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638-3646. [DOI] [PubMed] [Google Scholar]
- 4. Sledge GW, Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy – MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6:116-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turner NC, Liu Y, Zhu Z, et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2019;37:1169-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Leary B, Hrebien S, Morden JP, et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun. 2018;9:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bagegni N, Thomas S, Liu N, et al. Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib. Breast Cancer Res. 2017;19:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Odan N, Kikawa Y, Matsumoto H, et al. Real-world outcomes of treating advanced breast cancer patients with palbociclib: a multicenter retrospective cohort study in Japan-the KBCOG-14 study. Breast Cancer. 2020;14:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raspé E, Coulonval K, Pita JM, et al. CDK4 phosphorylation status and a linked gene expression profile predict sensitivity to palbociclib. EMBO Mol Med. 2017;9:1052-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Portman N, Milioli HH, Alexandrou S, et al. MDM2 inhibition in combination with endocrine therapy and CDK4/6 inhibition for the treatment of ER-positive breast cancer. Breast Cancer Res. 2020;22:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Formisano L, Lu Y, Servetto A, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. 2019;10:1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robbins P, Pinder S, de Klerk N, et al. Histological grading of breast carcinomas: a study of interobserver agreement. Hum Pathol. 1995;26:873-879. [DOI] [PubMed] [Google Scholar]
- 13. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155-168. [PubMed] [Google Scholar]
- 14. Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113:150-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10:4895-4900. [DOI] [PubMed] [Google Scholar]
- 17. el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987;139:2406-2413. [PubMed] [Google Scholar]
- 18. Chen Y, Chen K, Xiao X, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asano Y, Kashiwagi S, Onoda N, et al. Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancer. Ann Surg Oncol. 2016;23:1104-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Myojin M, Horimoto Y, Ito M, et al. Neutrophil-to-lymphocyte ratio and histological type might predict clinical responses to eriburin-based treatment in patients with metastatic breast cancer. Breast Cancer. 2020;27:732-738. [DOI] [PubMed] [Google Scholar]
- 21. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31:1623-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emens LA, Molinero L, Loi S, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the impassion 130 study. J Natl Cancer Inst. 2021;113:1005-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dieci MV, Barbieri E, Piacentini F, et al. Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. Ann Oncol. 2013;24:101-108. [DOI] [PubMed] [Google Scholar]
- 24. Amir E, Miller N, Geddie W, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thompson AM, Jordan LB, Quinlan P, et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. 2010;12:R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watanabe J. Reply to letters to the editor: discordance in estrogen receptor and change of Ki67 between primary site and metastatic site of recurrent breast cancer. Breast Cancer Res Treat. 2020;182:513-514. [DOI] [PubMed] [Google Scholar]
- 27. Horimoto Y, Tokuda E, Murakami F, et al. Analysis of circulating tumour cell and the epithelial mesenchymal transition (EMT) status during eribulin-based treatment in 22 patients with metastatic breast cancer: a pilot study. J Transl Med. 2018;16:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rugo HS, Finn RS, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174:719-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Battisti NML, Kingston B, King J, et al. Palbociclib and endocrine therapy in heavily pretreated hormone receptor-positive HER2-negative advanced breast cancer: the UK Compassionate Access Programme experience. Breast Cancer Res Treat. 2019;174:731-740. [DOI] [PubMed] [Google Scholar]
- 30. Turner NC, Finn RS, Martin M, et al. Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol. 2018;29:669-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Masuda N, Nishimura R, Takahashi M, et al. Palbociclib in combination with letrozole as first-line treatment for advanced breast cancer: a Japanese phase II study. Cancer Sci. 2018;109:803-813. [DOI] [PMC free article] [PubMed] [Google Scholar]