Abstract
Background
Regenerative medicine is the fastest developing branch of plastic surgery in recent times. Adipose tissue is one of the largest and most important sources in the body for stromal cells. Although mechanical isolation methods are both very popular and have many advantages, they still have no accepted protocols.
Objective
We developed new protocols called indication-based protocols (IPs) for standardization and new techniques called mechanical stromal-cell transfer (MEST) by using ultra-sharp blades and dilution of adipose tissue with different solutions (saline, Ringer and 5% Dextrose)
Methods & material: In order to obtain the desired physical structure (liquid, gel, solid) and the desired volume, four different types of IPs have been defined. Adipose tissue was prediluted with different solutions using 10 or 20 cc injectors in IPs 1 and 2, while condensed adipose tissue was used directly in IPs 3 and 4.
Results
In MEST, stromal cells were obtained from 100 mL of condensed fat using different IPs with 92% mean viability and cell counts of 26.80–91.90 × 106. Stromal cells can be obtained in the desired form and number of cells by using four different IPs.
Conclusion
Isolation of stromal cells by cutting fat with sharp blades will prevent the death of fat tissue and stromal cells and will allow high viability and cell count with our new technique. Predilution with different solutions: Diluting the condensed adipose tissue with the desired solutions (saline, Ringer or 5% Dextrose) before the adinizing process will provide even more stromal cells.
Lay Summary
Obtaining regenerative stromal cells from adipose tissue can be done by two methods: Enzymatic and mechanical. Mechanical methods have many advantages. Although mechanical stromal cell extraction from adipose tissue is very popular and many techniques have been described, there are still no accepted protocols, definition for the end product, and no consensus on the status of the stromal cells. In this study, stromal cells were obtained mechanically by using ultra-sharp blade systems, without exposing adipose tissue to blunt trauma. Thus, a higher number of cells and higher viability could be obtained. An “Indication based” protocol has been defined for the first time in order to obtain the desired number and status (solid, semi-solid, liquid)
end product. Diluting the condensed adipose tissue with the desired solutions (saline, Ringer or 5% Dextrose) before the adinizing process will provide even more stromal cells. This will provide an opportunity for clinicians to obtain and apply a stromal cell solution for different indications in different anatomical regions.
Keywords: Stromal cells, adipose tissue, stromal vascular fraction, mechanical, total stromal-cells, cell treatment
Introduction
Plastic surgery is perhaps the fastest developing specialty in all medical branches, scientifically and technologically. The greatest improvement in plastic surgery in the last 20 years has undoubtedly been in the field of regenerative science. Tissues, which are the infrastructure of the organs of our body, consist of two types of cells: parenchymal and stromal. While parenchymal cells are the cells that perform the main function of the organ, stromal cells are the main support and repair cells, and they are responsible for the repair of the tissue in case of any trauma, loss of function or injury due to aging. 1 In fact, one of the most important features of adipose tissue, in addition to its thermoregulation, shock absorption, and being the body's energy storage, is that it is the largest and most important regenerative cell source in the body. 2 This tissue is a type of loose connective tissue that contains an eclectic reservoir of cells, including immune cells, erythrocytes, progenitors, and stromal components. 3 The stroma is the area with support cells. 4 The use of regenerative cells from adipose tissue is not a new subject. The first applications which promoted healing by transferring the fat tissue particles into the wounds of the injured soldiers were made by Morrestin in WW1, 5 thus using the regenerative effect of fat tissue. In 1986 Jarrell 6 presented the effect of microvascular endothelial cells from adipose tissue in his study, but it was not until later that this issue was popularized by Zuk et al. in 2001. 7 They showed that fat tissue is very important and the largest mesenchymal stem / stromal cell source of body. Stromal cells from the adipose tissue for regenerative purposes are obtained by two methods: enzymatic and mechanical. Until recently, the gold standard was the use of enzymes in the extraction of stromal cells from adipose tissue. The enzyme destroyed the dense bonds in the adipose tissue, and stromal cells were obtained by centrifugation. 8 This stromal cell cocktail, including fat stem cells, was called stromal vascular fraction (SVF). The term stromal vascular fraction (SVF) is used herein only for stromal cells obtained by enzymatic procedures. Recently, Tonnard et al., who changed our perspective on the isolation of stromal cells from adipose tissue, offered a definition of nanofat in their 2013 study. 4 Working even more superficially with still finer sharp needles (27 gauge), the harvested fat was mechanically emulsified and filtered until a liquid suspension was obtained. They called this “nanofat”. The term SVF is not accurate for mechanically-derived stromal cells for many reasons, therefore, many authors instead prefer to use the term nanofat for stromal cells obtained mechanically. The terms SVF and nanofat identify two completely different final tissues and cellular samples derived from adipose tissue: SVF indicates a complex pool of heterogeneous nucleated and non-nucleated cells (e.g. pre-adipocytes, pericytes, mesenchy-mal progenitors, endothelial cells, fibroblasts, mesenchymal T cells, M2 macrophages, and leukocytes), while the term nanofat refers to a commercial trademark of a device for filtering and emulsifying adipose tissue for reinjection into different body areas without any type of isolation process. Thus, SVF and nanofat express completely different structures and preparations. Although the mechanical isolation of stromal cells from adipose tissue is very popular, the biggest problem is that there are many distinct products and no accepted protocol. Various products and protocols lead to different results in both numbers and quality.9–18 Even the physical structure of the final product varies. In some protocols, total stromal cells were obtained only in solid form, in others, in gel and liquid form. As with any surgical technique or medical procedure, there must be accepted protocols for the standardization of results achieved from the mechanical isolation of stromal cells from adipose tissue. For this purpose, we developed new protocols called indication-based protocols (IPs) for the standardization of protocols and end products, and a new technique called mechanical stromal-cell transfer (MEST) for better results. The most criticized aspect of mechanical methods is excessive blunt pressure applied to adipose tissue. 12 This pressure causes the death of not only adipose tissue but also stromal cells. Therefore, in the method we developed, we aimed to cut the adipose tissue with sharp knives without applying excessive blunt pressure.
Indication-based protocols (IPs) are protocols developed to determine the desired physical structure and amount of the final product in the process of mechanical stromal cell isolation form adipose tissue, by directly processing the condensed adipose tissue with or without adding the desired fluid.
Material method
This study was conducted according to the standards of good medical practice (ICH-E6) and the principles of the Declaration of Helsinki. Regarding the use of human tissue specimens, our Institutional Review Board (IRB) are responsible for determining whether or not informed consent is required from the subjects from whom the specimen were obtained. Informed consent was obtained from subjects in accordance with the regulations of the Institutional Review Board.
All patients were provided detailed information preoperatively and gave written consent for all surgical procedures, anesthesia, intraoperative video recording, and photography. In addition, a written consent form was obtained from the patients stating that they willingly donated their adipose tissue for laboratory analysis. In this study, a patented CE marking and ISO 13485 certified blade system was used, and rules of minimal manipulation were followed. No enzymes or similar chemicals were used, and the structure of the fat tissue was not altered.
MEST technique and different types of IPs were tested with abdominoplasty specimens from 12 voluntary female cases.
Preparing of MEST
We used a patented very sharp blade system (Adinizer, BSLrest, South Korea) in different diameters, both while bringing the adipocytes to the size we wanted and in mechanically obtaining stromal cells from adipose tissue (Figure 1(a), (b) and (c)). In the MEST procedure, there are four basic steps, each of which has its own philosophy:
Lipoharvesting: Adipose tissue responds to pressure by cell death; therefore, excess pressure should be avoided in both lipoharvesting and lipofilling processes. We used a 2.8 mm diameter cannula with a blunt tip and four eccentric holes. The fat tissue was manually harvested using 20 mL Tara-lock system (BSL, South Korea) with BD injector luer-lock,
Lipo-condensation: Condensation procedures aim to increase the relative number of stromal cells per tissue volume simply by eliminating some components, such as adipocytes, red blood cells, fat, and aqueous fractions, which are present in the lipoaspirate. The principal methods for adipose tissue condensation are gravity-based decantation, filtration, and centrifugation. For our purposes, the centrifuge method was preferred.,
-
Adinizing according to Indication based protocols (IPS): In order to obtain the desired physical structure (liquid, gel, solid) and the desired volume, four different types of IPs have been defined. Adipose tissue was prediluted with saline or Ringer solution or 5% Dextrose solution using 10 or 20 cc injectors in IPs 1 and 2, while condensed adipose tissue was used directly in IPs 3 and 4. In accordance with the selected IPs, 10 or 20 cc luer-lock injectors were used. Details of IPs is presented in Table 1
There is no term in the literature that describes the process of cutting fat tissue with blades. “Adinizing” is a new term and means cutting the fats with ultra-sharp blades to bring the fat tissue to the desired size and to separate the stromal cells from the parenchymal cells in adipose tissue. Patented adinizer blade wheels are used for this process. These start from 4000 μ and alternate wheels include blades of 2,400, 1,200, 600, 400, 200, and 100-μ sizes. Starting the adinizing process with a small diameter knife will increase the amount of blunt trauma or positive pressure per unit of adipose tissue. Therefore, the adinizing process starts with 4000 μ—the red smart wheel—and decreasing diameters are used in descending order through the blades with 2400, 1200, 600, 400, 200, and 100 μ diameters, to ensure that the minimum pressure is applied to the adipose tissue. We also defined and got patent a new approach as IPs for having better and suitable end product for different indications,
Stromal Cell isolation: After the adinizing process, the product is centrifuged for the last time at 1200 g for 6 min, as a result of this process, 2 separate layers are formed at the bottom depending on the density (video- study). These layers are stromal-cell solution (SCS) at the bottom, stromal-cell aggregate (SCA) above it. The bottom layer and just above SCS and SCA were taken for stromal-cell transfer and used for regenerative purposes. The determination of total stromal cells (TOST) was used for these two plates. Since the term SVF is a specific definition for enzyme stromal cell isolation, and there is no specific definition for accepted mechanical stromal cell isolation, we preferred to use the term TOST instead of SVF.
Figure 1.
(a) Adinizers: ultra-sharp blade system. Diameter of blades start from 4000 μ to 100 μ. (b) Schematic view of cross section of Adinizer two-sided blades. (c) View of blades of 100 μ Adinizer under microscope (×1000 magnification).
Table 1.
Summary of indication-based protocols (IPs).
| IP's # | Total Volume | Suggested Fat Volume | Suggested Predilution Fluid (saline or Ringer solution or 5% Dextrose) Volume | Desired Product | Desired Volume |
|---|---|---|---|---|---|
| 1 | 10 cc | 5 cc | 5 cc | Solution | 5–6 cc |
| 2 | 20 cc | 10 cc | 10 cc | Solution | 8–12 cc |
| 3 | 10 cc | 10 cc | 0 | Gel/dense solution | 1–2 cc |
| 4 | 20 cc | 20 cc | 0 | Gel/dense solution | 3–4 cc |
The final products containing stromal cells obtained were evaluated simultaneously with a Luna-stem device in the operating room environment for viability and cell number. Final products were transferred to a sterile bag and transported to the cell therapy unit for further analysis. Extensive characterization tests were performed to obtain data from the adinized products of adipose tissue, and the following analyses were performed on different regenerative products in each IP (Table 2).
Table 2.
Comparison of IPs for total volume and end-product.
| IP's | Condensed fat volume (mL) | Pre-Adinizing Total Volume (mL) | Post-Adinizing Total Volume (mL) | TOST-cell volume |
|---|---|---|---|---|
| IP’s 1 (saline) | 5 | 10 | 9 | 5.2 ± 0.8 |
| IP's1 (Ringer) | 5 | 10 | 9 | 5.1 ± 0.8 |
| IP's1 (Dextrose) | 5 | 10 | 9 | 5.0 ± 0.8 |
| IP's 2 (saline) | 10 | 20 | 19 | 11.0 ± 1.2 |
| IP's 2 (Ringer) | 10 | 20 | 18 | 10.2 ± 1.0 |
| IP's 2 (Dextrose) | 10 | 20 | 18 | 10.0 ± 2.2 |
| IP's 3 | 10 | 10 | 8 | 2.1 ± 0.3 |
| IP's 4 | 20 | 20 | 18 | 4.6 ± 1.2 |
Freshly isolated cells from different IPs were examined for cell count, viability and surface molecule expression using flow cytometry. The cells were neutralized, washed, filtered, counted, and labeled with the following dyes and antibodies : 7-AAD (BD Pharmingen, Franklin Lakes, NJ, USA), monoclonal antibodies (MAb), from BD Biosciences Pharmigen (San Jose, CA, USA) unless other stated, conjugated to fluorochromes were used: CD31- APC (R&D Systems, McKinley Place, Minneapolis, USA), CD34-P-Cy7, CD45-APC-Cy7, CD90-FITC, CD146-PE (R&D Systems, McKinley Place,Minneapolis, USA). TOST cells were analyzed using a NAVIOS flow cytometer (Beckman Coulter, Miami, FL, USA).
Viability and nucleated cell counts
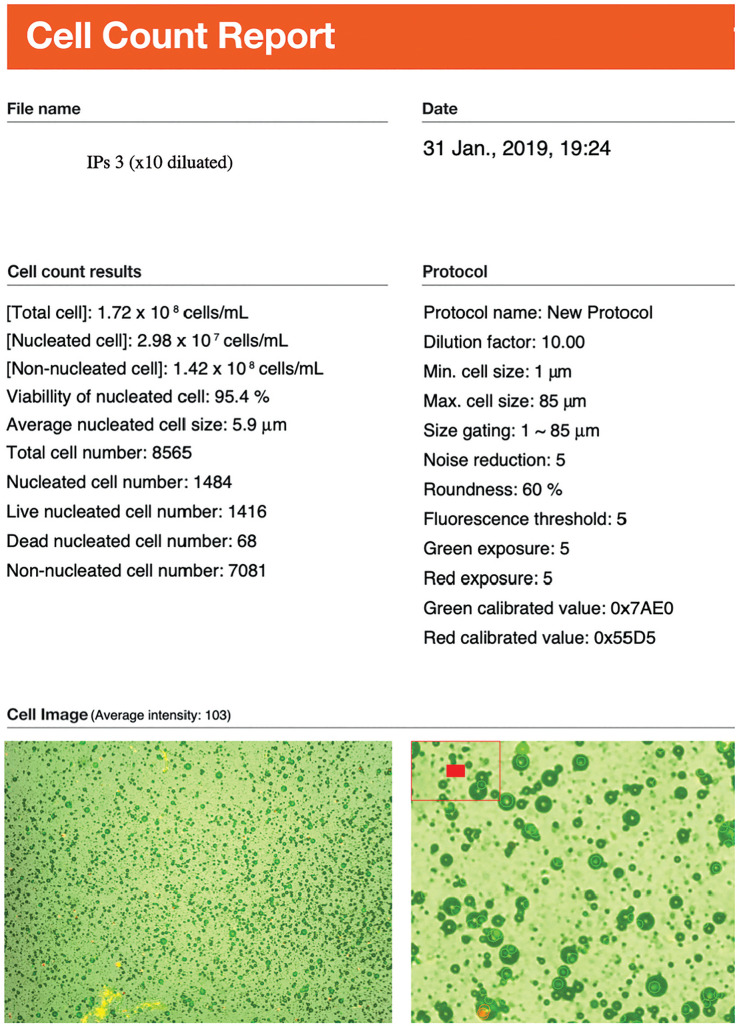
Total viable nucleated cell recovery and viability percentage were determined using the LunaStem Automated Fluorescence Cell Counter device (Logos Biosystems, South Korea) (Figure 2).
Figure 2.
Cell amount and viability results of IPs3 by LunaStem Automated Fluorescence Cell Counter device (Logos Biosystems, South Korea). Since TOST was solid form in IPs3, we have diluated 1/ 10 ratio. Nucleated cell amount should be accepted as 2.98 × 106/mL.
Flow cytometry analysis of TOST cell subsets
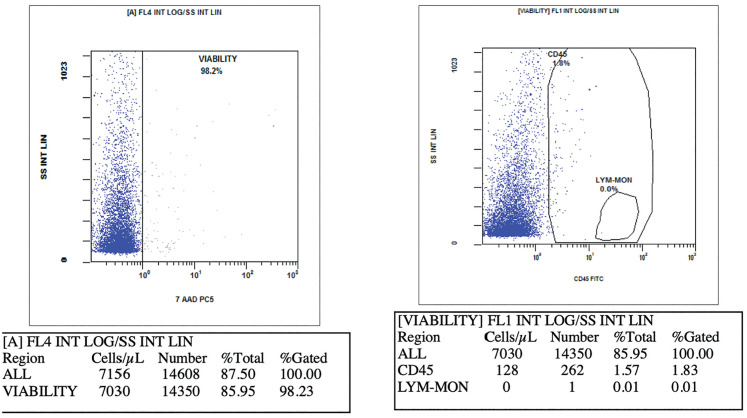
Characterization of the SVF cell subpopulations was performed according to the recommendations of the International Federation of Adipose Therapeutics and Sciences (IFATS) and the International Society for Cellular Therapy (ISCT). Briefly, TOST suspension was digested with DNase I 10U/mL (Roche Diagnostics, Indianapolis, Indiana, USA) in DPBS Ca++/Mg++ free medium containing 0.1 mM EDTA, 25 mM Hepes, 1%FCS (Capricon-Scientific, Ebsdorfergrund, Germany) for 15 min at 37°C and filtered through a 100μm nylon cell strainer to eliminate the majority of cell aggregates. Cells were centrifuged, re-suspended and labeled 20 min at 4°C with the following fluorochrome-conjugated antibodies described above. Red blood cells were lysed in NH4Cl for 10 min at 4°C before cells were centrifuged and re-suspended in DPBS Ca++/Mg++. NucBlue (Thermo Fisher Scientific, Waltham, Massachusetts, USA), allowing discrimination of viable and dead cells, was added for 5 min prior to flow cytometry analysis on a NAVIOS flow cytometer (Beckman Coulter, Miami, FL, USA). Cell counting and viability were also performed as flow cytometers (Figure 3).
Figure 3.
Cell amount and viability results of IPs3 by NAVIOS flow cytometer (Beckman Coulter, Miami, FL, USA).
After adinizing, a sample was taken, and histopathological examination was performed to examine the fat tissue.
Comparison of results of IPs 1 and 2 subgroups and all IP groups (the data analysis was carried out using IBM SPSS Statistics for Windows [version 21.0; IBM Corp., Armonk, NY, USA]). The descriptive statistics were given as mean ± standard deviation. The normal distribution of the numerical variables was determined by using the Shapiro-Wilk normality test. If the data complied with a normal distribution, the statistical differences between the groups were evaluated using the one-way analysis of variance and post hoc tests. If the data did not comply with a normal distribution Mann-Whitney U tests were used. A P value of <0.05 was considered to be statistically significant
Results
The adipose tissue used in the study was obtained from 12 consecutive female patients who underwent abdominoplasty. Patients were non-obese pattern, aged between 36 and 51 years (mean age, 42 years) and a mean body mass index of 28 kg/m2 (range: 20–33 kg/m2).
Four different IPs were applied to each abdominoplasty material taken from healthy volunteers as soon as the tissue was excised. 80 cc lipoaspirate has been harvested with 20 cc Tara-lock system. After condensation with centrifuge, as presented in Table 1, 5 cc of fat is mixed with 5 cc saline or Ringer solution or 5% Dextrose solution in IPs1 while obtaining a 10 cc emulsion, while in IPs2, 10 cc fat is mixed with 10 cc saline or Ringer solution or 5% Dextrose solution and 20 cc emulsion is obtained. 10 cc and 20 cc condensed fat is used in IPs 3 and 4, without mixing the saline and fat tissue. 4.4–6.0 mL of TOST was obtained using 5 mL of fat and 5 mL of saline in IPS1; 4.3–5.9 mL of TOST was obtained using 5 mL of fat and 5 mL of Ringer in IPS1 and 4.2–5.8 mL of TOST was obtained using 5 mL of fat and 5 mL of 5%Dextrose in IPS1. As flow cytometric, in IPs1 saline group, average of 940.000 (765.000–1.400.000) cells were obtained in 1 mL; in IPs1 Ringer group, average of 890.000 (752.000–1.300.000) cells were obtained in 1 mL and in IPs1 5%Dextrose group, average of 880.000 (750.000–1.300.000) cells were obtained in 1 mL. When these cell numbers were measured using LUNA dual fluoroscopy, an average of 890.000 (621.000–1.240.000) cells were obtained in IPs saline group and an average of 870.000 (618.000–1.200.000) cells were obtained in IPs1 Ringer group and an average of 790.000 (620.000–1.100.000) cells were obtained in IPs 5% Dextrose group. Using 10 mL fat and 10 mL fluid in IPS2 (saline), TOST was obtained between 9.8–12.2 mL, and in IPs2 (Ringer) TOST was obtained between 9.2–11.2 mL and in IPs (5% Dextrose) TOST was obtained between 7.8–12.2 mL. Average 910.000 (700.000–1.380.000) cells were obtained in 1 mL as flow cytometric in IPs2 (saline) and 900.000 (760.000–1.120.000) cells were obtained in 1 mL as flow cytometric in IPs2 (Ringer) and 880.000 (700.000–1.000.000) cells were obtained in 1 mL as flow cytometric in IPs2 (5% Dextrose). When these cell numbers were measured using LUNA dual fluoroscopy, an average of 790,000 (650,000–1,100,000) cells were obtained in IPs (saline) and average of 770,000 (660,000–1,000,000) cells were obtained in IPs (Ringer) and average of 760,000 (640,000–1,000,000) cells were obtained in IPs (5% Dextrose). Although more cells, more viability and TOST volume were obtained in both IPs 1 and IPs 2 in the saline group compared to Ringer's and 5% Dextrose solution, they were not statistically significant (0.913, 0.831). In IPs 3, only 10 mL of condensed fat was used and TOST was obtained between 1.8–2.4 mL. An average of 1.660.000 (1.200.000–1.880.000) cells was obtained in 1 mL as flow cytometric. When these cell numbers were measured using LUNA dual fluoroscopy, an average of 1,560,000 (1,150,000–1,900,000) cells were obtained. In IPs 3 without any solution, more statistically significant cells were obtained compared to all groups in IPs 1(<0.001). Finally, in IPs 4, only 20 mL of condensed fat was used and TOST was obtained between 3.4–5.8 mL. An average of 1.490.000 (1.100.000–1.950.000) cells were obtained in 1 mL as flow cytometric. When these cell numbers were measured using LUNA dual fluoroscopy, an average of 1.380.000 (1.050.000–1.800.000) cells were obtained. Similarly, a more statistically significant number of cells were obtained in IPs 4 without any solution compared to all groups in IPs 2 (<0.001). In order to be able to compare with other publications presented in the literature, the rates that can be obtained from 100 mL condensed fat were also calculated. Tables 3 and 4 show the volume, cell count, viability results of the end product in each IPs. Physically, IPs 1 and 2 were obtained liquid form and 3 and 4 solid form TOST (Figure 4).
Table 3.
Viability and total number of nuclear cells per milliliter, total and 100 mL after adinizing for each IPS by dual -fluoroscopy device.
| IP's | Viability % | NC (Average) 106 per mL | Total NC (Average) 106 | 100 mL Condensed Fat TNC 106 |
|---|---|---|---|---|
| IP's1 (saline) | 93 ± 2 | 0.89 | 4.26 | 85.20 |
| IP's1(Ringer) | 92 ± 1 | 0.87 | 3.86 | 81.80 |
| IP's1(Dextrose) | 90 ± 3 | 0.79 | 3.71 | 78.60 |
| IP's2(saline) | 93 ± 2 | 0.79 | 8.26 | 82.60 |
| IP's2(Ringer) | 92 ± 2 | 0.77 | 8.02 | 79.80 |
| IP's2(Dextrose) | 91 ± 2 | 0.76 | 7.94 | 77.60 |
| IP's 3 | 94 ± 2 | 1.56 | 2.80 | 28.0 |
| IP's 4 | 94 ± 2 | 1.38 | 5.36 | 26.80 |
Table 4.
Viability and total number of nuclear cells per milliliter, total and 100 mL after adinizing for each IPS by flow cytometry.
| IP's | Viability % | NC (Average) 106 per mL | TNC (Average) 106 | 100 mL Condensed Fat TNC 106 |
|---|---|---|---|---|
| IP's1(saline) | 94 ± 2 | 0.94 | 4.51 | 90.24 |
| IP's1(Ringer) | 93 ± 2 | 0.89 | 4.32 | 88.24 |
| IP's1(Dextrose) | 91 ± 2 | 0.88 | 4.23 | 85.60 |
| IP's2(saline) | 95 ± 2 | 0.91 | 9.19 | 91.90 |
| IP's2(Ringer) | 94 ± 2 | 0.90 | 8.90 | 88.70 |
| IP's2(Dextrose) | 94 ± 2 | 0.88 | 8.60 | 86.20 |
| IP's 3 | 98 ± 6 | 1.66 | 2.98 | 29.80 |
| IP's 4 | 94 ± 4 | 1.49 | 5.79 | 28.95 |
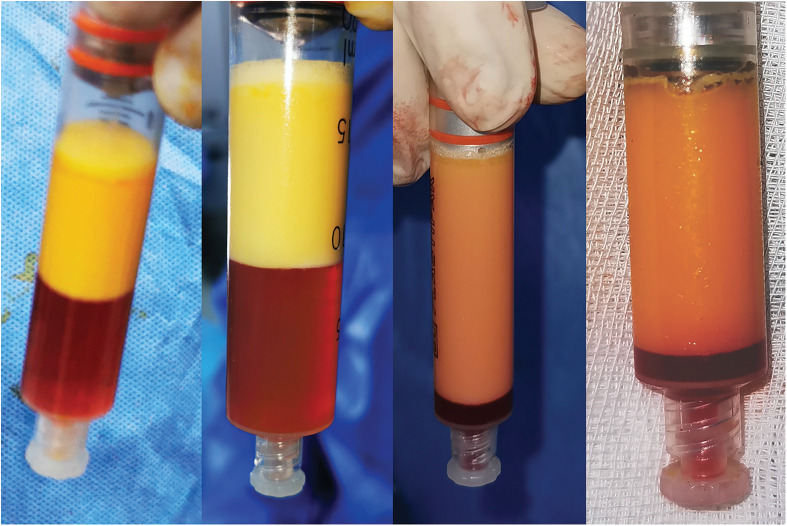
Figure 4.
Final view of each IPs.
IPs 2 is denser than 1, IPs 3 is in gel form and IPs 4 is in very solid form. As a result of all processes in IPs 1 and 2, four layers are obtained (Figure 4). These layers are stromal-cell solution (SCS) at the bottom, stromal-cell aggregate (SCA) above it, adinized fat tissue layer above that, and triglycerides (TG) at the top. Adinized adipose tissue was evaluated with hematoxylin eosin and the intact structure of these cells was shown histopathologically (Figure 5). However, in IPs 3 and 4, there will be no adipose tissue, which is the second layer from the top, there are residues of TG and shredded adipose tissue at the top, and the lower two layers have a denser and more voluminous SCA and less voluminous SCS (Figure 6).
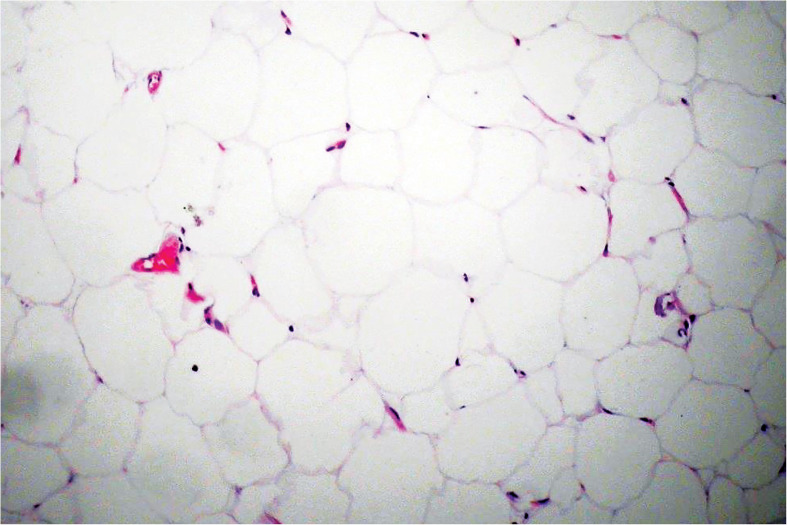
Figure 5.
Histopathological assessment of Adinized adipose tissue after adinized 100 μ blades and centrifugation in 1200 g, 6 min by Hematoxylin-Eosin staining. Cell integrity is still stable.
Figure 6.
(a) View of IPs1 after all process. There are four layers and stable adipose tissue above the TOST. (b) View of IPs3 after all process. There are 3 layers and there is stable adipose tissue above the TOST.
Discussion
Nanofat, the father of mechanical stromal cell isolation, was described by Tonnard in 2013. 4 Stromal cell isolation from adipose tissue mechanically has seen increasing popularity since Tonnards study, and many different techniques and tools have been presented.4,9–18 In original nanofat technique, Tonnard harvested fat tissue with 2.4 mm cannulas with 1 mm holes. Then, the isolated lipoaspirate was rinsed and adipose tissue was emulsified with 2.4 and 1.2 mm connectors, respectively with 30 times back-forth movements between two syringes. Finally, it was filtered using 600–400 μ filters. Although the term nanofat has become popular since 2013, this new concept has been criticized in three aspects: First, in terms of the concept of “nano”, a nanometer is one-billionth of a meter, and dimensions between approximately 1 and 100 nanometers are known as the nanoscale, but in general, nanofat is thought of as fat parcel sizes of 600 μ. 19 Second, in terms of “fat”, Stuzin, who discussed Tonnard's paper, stated that the most intriguing point in the article is that the substance the authors term nanofat is not fat at all. 20 After histologic examination of their suspension, the authors realized that in processing the nanofat, the normal fat structure was destroyed. 20 Finally, they preferred using the term “emulsification” for the process of the mechanical isolation of stromal cells from adipose tissue in the original study of Tonnard in 2013. 4 But emulsification is to force two immiscible liquids to combine in a suspension—substances like fat and water, which cannot dissolve in each other to form a uniform, homogenous solution. In many publications to date, shifting the fat between two 10-cc syringes connected to each other by a female-to-female luer-lock connector process has been defined as emulsification of the fat.4,9–18 The reason for such a large number of different techniques and tools is the advantages of mechanical methods. Mechanical methods have many advantages over enzymatic methods.12,21 The enzymatic disruption of adipose tissue results in a single-cell suspension in which all cell-cell communications are fully disrupted, and the extracellular matrix is digested; adipocytes are destroyed too. After mechanical isolation, however, adipocytes are also destroyed, but intercellular connections and cell–extracellular matrix connections remain intact. 14 Since sharp blades were used in our study, the structure of adipocytes is also preserved. This has been shown histopathologically. Even after the last centrifuge, there is still intact adipose tissue, which is used for soft tissue augmentation. Moreover, the extracellular matrix, which is an important reservoir of growth factors and acts as an instructive scaffold in the regenerative processes, also remains intact, in contrast to enzymatically dissociated lipoaspirate. When adipose tissue is enzymatically digested, the architecture and instructive capacity of the stromal tissue are also fully destroyed, although the isolated stromal tissue cells will survive. 14 More effective stromal cells can be obtained by mechanical methods in greater numbers and with more compositions for wound healing and regeneration. 22 Mechanical shear stress is always created in these techniques, which may lead to the up-regulation of multipotent and pluripotent markers that connote regenerative capacity. 23 Compared to traditional enzymatic methods, similar or higher numbers of cells can be obtained in mechanical separation techniques while using 10 times less fat tissue. 22 In addition, their natural matrix niches are preserved in the mechanical aggregates (nanofat), which promotes cell viability, proliferation, and differentiation. 22 Moreover, obtaining SVF enzymatically is time-consuming and expensive, requiring equipment and personnel, and above all, it is an application that is classified as a biological drug by authorities, such as the FDA and EMA, meaning that conditions of current good manufacturing protocols (cGMP), current good laboratory protocols (cGLP) must be met, which is impossible for many hospitals and doctors. 24 Unfortunately, unlike for the enzymatic approach, there are no established standards or protocols for mechanical methods. Perhaps the most important problem in stromal-cell isolation by mechanical means is that the final product obtained is not standard. First, the intended end product must be correctly identified. Because the term SVF is used for a fat tissue-derived regenerative cell cocktail obtained using enzymes such as collagenase, 8 the term is unsuitable for mechanically-derived stromal cells for many reasons. Although regenerative cells originating from adipose tissue mechanically are often referred to as “nanofat,” many alternatives are also present in the literature, including super micro-fat, 9 fat paste, 10 ultra-micro-fat, 10 SVF gel, 11 non-enzymatic SVF, 12 fluid fat, 13 tissue-like SVF, 14 mechanical SVF, 15 fracto-fat, 16 stem-like cells, 17 cell aggregates, 16 fat press, 18 human adipose liquid gel. 25 Since the main purpose of mechanical methods is to protect all the stromal cells, we think the final product should be called total stromal cells (TOST). We recommend using this term for mechanically-derived stromal cells rather than SVF or nanofat. There is no consensus on not only the definition but also the quantity and quality of the product isolated. The reason for this is that mechanical approaches are much simpler than enzymatic, and thus, many methods have been described, but unfortunately there are no protocols as there are for enzymatic approaches. Since the extra-cellular matrix (ECM) and cell-cell connections are maintained, a nearly solid product is obtained, both in liquid and dense mass. However, in the enzyme method, SVF, the final product, is always in the same liquid form. With the IPs we have defined, TOST can be obtained in any quantity and volume that is suitable for the desired application. This is a great advantage, especially since its liquid form is SVF-like. Unlike the decomposing enzyme, stromal cells and ECM are not destroyed but maximally protected in mechanical methods. Widely differing results have been reported regarding the viability and number of cells in mechanical stromal-cell production.4,22,26,27 In our technique, a new protocol has been defined and patented to obtain stromal cells with different compositions and physical structures for various indications in mechanical techniques—an indication-based protocols (IPs) (Table 1). Using this protocol, we developed varying amounts of stromal cells for different indications, especially in the solution form. Four separate IPs were defined. The aim is the isolation of the bottom stromal-cell solution and the cell aggregate, and the final product, obtained by mixing these two layers together, is called the total stromal cells (TOST). Adipose tissue was prediluted with saline using 10 or 20 cc injectors in IPs 1 and 2, while condensed adipose tissue was used directly in IPs 3 and 4. In accordance with the selected IPs, 10 or 20 cc luer-lock injectors were used. An injector with condensed fat was placed on one end of the adinizer disk, an empty injector was placed on the other end, and the fat tissues were cut with sharp blades in the adinizer under minimal pressure with back-and-forth movements. The back-and-forth movement was made 25 times on average, at least 20 and up to 30. While starting the process of mechanically cutting the fat, pressure can be felt in the injectors between the researcher's fingers, and this pressure subsides after approximately 20 passes. The relief of the pressure indicates that sufficient cuts have been made, and the process is terminated with a small cut having been made. Adinizing was first performed with a 4000-μ adinizer; after approximately 25 passes, the cutting process was continued with the next-smaller diameter blades. The term adinizing, which we defined above, is used for the first time in the literature; it defines the process of cutting fat tissue without blunt pressure, with sharp blades for reducing grafts to the desired diameter and separating the parenchymal and stromal cells in adipose tissue without using enzymes. The separation process with sharp blades will not only ensure the separation of stromal cells but also adipocyte-derived stem cells (ASCs). 26 When this work is done with an enzyme, such as collagenase, destruction in both ECM and stromal regenerative cells occurs. 26 We developed new protocols termed indication based protocol (IPs) for standardization and a new technique called mechanical stromal-cell transfer (MEST). All known techniques for adipose tissue manipulation require infiltration, the harvesting of adipose tissue, processing the samples, an isolation phase, and reinjection. If these steps are realized following known technical protocols, the final results might be unavoidably different for types of cells, number, vitality, viability, and characterization. Nevertheless, the superiority of mechanical techniques over enzymatic methods is due to their maximal protection of stromal structures and because, according to the criteria of flow cytometry characterization in the guidelines of joint statements from IFATS and ISCT, more stromal cells are obtained. Obtaining stromal cells from adipose tissue mechanically has been very popular recently, there are studies in the literature related to many devices.22,26,28 Very different results have been reported regarding the viability and number of cells in mechanical stromal-cell production.4,22,24,29 As a matter of fact the phenotypic characterization of adipose derived tissue cell precursors can show significant differences from one patient to another and from one anatomical area to another. Literature suggests that, except for donor areas, sex, race, age, body structure and fatty component profile (hypo/normo/hypertrophic AT) can be strong determinants of the quality of the ASCs. Flow cytometry data revealed a high variability among patients and anatomic areas of CD105 (+)CD45(–) cells. 30 It is a common finding, for example, that younger patients own a pool of ASCs precursors with a greater proliferative capacity (and higher phenotypic expression for surface markers) than those from older patients. In Tonnard's study, 4 the first research published on this subject, in measurements using flow cytometry, 1,975,000 cells were reported in 100 mL of fat, while in our study the lowest rate was found to be 26,800,000 and the highest 91,900,000. In many other studies, very different numbers were presented,4,22,26,28 even in several studies using the same product.27,29,31 More recently, Cohen et al. published a comparison of two different commercial mechanical stromal cell isolation products. 32 The first one was the Tulip NanoTransfer kit (Tulip Medical, Inc., San Diego, CA), which consists of 2.4,1.4 and 1.2 mm connectors and 400 and 600 μ two-layered filters. The other one was the LipocubeNano device which consisted of four ports and a single 500 μ filter. They found that the LipocubeNano produced a cell count of 2,240,000 cells per cc and cell viability of 96.75% and Tulip's nanoTransfer method resulted in 1,440,000 cells per cc with a cell viability of 96.05%. Both systems perform mechanical separation with blunt edge filters, not a sharp blade system. However, in the adinizer and in both of our techniques, the main strategy is to avoid excessive blunt pressure by cutting the fat with sharp blades. The adinizer plays an important role in the sharp blade edges (Figure 1) and protrusions of through-holes is the biggest difference from Lipocube and other productors. Various methods are used to verify the number and viability of the stromal cells obtained. These include counting chambers/hemocytometers, automated cell counters, Coulter counters, flow cytometry, and DNA weight analysis devices.4,22,29 Although the most widely accepted is flow cytometry, its application is not practical in operating room conditions. Automated cell counters developed for enzymatic methods give very practical and effective results that are generally developed for enzymatic methods. There are no studies in the literature confirming the results and reliability of these devices, especially obtaining stromal cells mechanically. In this study, we compared dual fluoroscopy, a practical device, with flow cytometry. The results were similar between the two. Although better results are obtained in flow cytometry, we think that particle dual fluoroscopy devices can also be used as a guide for the surgeon.
In their study, Sesé et al. proved that the main stromal cells are in the adipose tissue excreted in enzymatic methods. 22 However, this fat tissue had to be discarded because it contained tissue enzymes. It is unclear how many stromal cells should be given to which tissue, but Sesé et al. described the cell dose. 22 The concept of standardizing different types of cellular derivates, tailormade and adapted to different types of anatomic areas and clinical applications for different specialties was first described by Zocchi. 33 The most detailed study on the clinical uses of stromal cells obtained mechanically was recently published by Ghiasloo et al. 34 Researchers scanned 4505 articles and created a database of 1458 diseases. One of the most interesting results of this study is that it mentioned 10 modifications besides the nanofat concept for mechanical stromal acquisition. Regarding clinical applications of stromal cells obtained mechanically, their review found eight separate studies on the quality and regeneration of skin, six on chronic wounds, diabetic foot and complications, and five for scar treatment in addition to those on knee osteo arthritis (OA), Achilles tendinopathy, temporomandibular joint (TMJ) dysfunction, and perineal fistula. They also reported their use in the treatment of vocal cord scars and paralysis, critical limb ischemia, androgenic alopecia, and migraine. It can be expected that, due to its potential use in such a broad range of treatments, TOST will be required in different qualities and quantities. However, although there is no clear protocol on the number of cells and volume of stromal cells to be applied to these various clinical states, each indication contains its specific case. Clinical applications of mechanically-isolated stromal cells will become more common with time; for now, the volume and number of cells used in the same SVF applications can be referenced. TOST will offer more types of stromal cells than SVF. For example, the concept accepted in knee OA is 6 ml and one million cells for each knee. 35 With IP 2, this volume and cell amount can be achieved in a very short time under local anesthesia with the MEST technique using only 10 ml of condensed fat. However, for TOST to be applied to vocal cords it has to be in denser form and much lower volume (video-clinical). Treatment of Peyronie's disease using SVF requires 50 cc fat harvesting and stromal-cell isolation using enzymes in GMP and GLP standards. 36 The same process can be done with IP 1 in a very short time and quite simply using only 5 cc of condensed fat under standard conditions. Similarly, in the treatment of erectile dysfunction, SVF is applied via the intra-cavernous route with 8.4–37.2 million regenerative cells. 37 This can be done very easily with IP 2 under local anesthesia with a higher number of cells. IPs allow different approaches for both the desired number of cells and the desired end product form. For example, if stromal cells will be applied to the face or scalp with needling only in the solution form, IP 1 or 2 is preferred, depending on the amount of fluid required, while IP 3 or 4 is preferred if the fat will be used to increase tissue retention. With IP 1, approximately 5 cc of liquid TOST can be obtained and applied to the whole face with a hydro-roller device or by needling in the nappage style, if desired. With IP 2, approximately 10 cc of TOST in liquid form can be obtained, which will provide sufficient volume and number of cells for penile applications in the treatment of ED or for the treatment of knee OA after a 45-μ filter. About 2 cc of TOST is obtained in IP 3 in a dense, stiffer form. This can be used successfully in scar treatment. Finally, about 4 cc of TOST is obtained in solid form in IP 4, which is an excellent source for cell-enriched fat grafts. It can be ideal for high-volume fat applications, such as breast reconstruction. Our results show that, by cutting the adipose tissue very gently with sharp blades without blunt pressure and without killing adipose tissue, both the desired size and the fat graft can be prepared in the same session, in many ways the stromal cells obtained can be superior to those obtained with enzymatic methods. Mechanical shear stress always occurs mechanically in the cell acquisition, which may lead to up-regulation of the multipotent and pluripotent markers of regenerative capacity. 37 Mechanical disaggregation requires 10 times less fat tissue as the starting material to provide a similar or even higher cell dose, compared with conventional enzymatic SVF isolation. 22 In addition to enhanced cell yield, mechanically disrupted cell aggregates (nanofat) remain attached to their natural matrix niche, which has been shown to promote cell viability, proliferation, and differentiation. 22 Even after the last centrifuge in IPs 1 and 2, the adipose tissue remained intact, which demonstrates how gentle this procedure is. In addition, the fat graft can be used successfully even in areas with the finest skin, such as the periorbital region, without unsightly visibility. In this study, our final product, TOST, was digested with DNAase enzyme in the subset analysis of stromal cells. Cell clumping can result in poor sort purity when sorted target cells are attached to non-target cells, and poor recovery when coincident aborts exclude all clumped cells. DNA from lysed cells in the medium can cause cells to clump. However, evaluation without using any enzyme or chemical product can lead to more meaningful results. Adipose tissue derived stromal cells should be evaluated with further studies in fresh tissue without enzyme.
Finally, in this study, mechanical stromal cells were obtained by diluting adipose tissue with different solutions (saline, Ringer, 5% Dextrose) for the first time in the literature. Although both cell count and viability were obtained in saline and then Ringer and 5% Dextrose less in all groups, all results were found to be higher than the fat group not treated with liquid. Different types of solutions can be used for different purposes in different anatomical areas. For example, while Ringer is mostly preferred in the joint, saline is preferred in wound applications. Thanks to this study, the practitioners were guided about the stromal cells obtained in each solution.
Conclusion
In our study, unique in the literature to date, indication-based protocols were defined and stromal cells were obtained in various cell numbers and volumes for different indications with different solutions (saline, Ringer and 5% Dextrose). We believe that predilution leads to better cutting of fat tissue and better isolation of stromal cells. We speculate that these effects can be explained by not only the difference in density but also the interaction and polarity between adipose tissue/saline. Adipocytes have no positive and negative charged points—the charge distribution is equal, indicating that they are nonpolar. Molecules that are nonpolar do not dissolve well in polar structures such as water; they tend to repel each other and remain separated, even when shaken vigorously. 38 However, mesenchymal stromal cells respond to superficial electric charges, unlike adipocytes. 39 When evaluated molecularly, salt dissolves in water due to its electrical charges and the polarity of both water and salt compounds; there are positive and negative charges on the opposite sides of the molecule. Due to the presence of electrical charges, bonds in salt compounds are called ionic bonds; while the sodium ions are positively charged, chloride ions are negatively charged. 40 With the back-and-forth movements described above, the stromal cells are released when the adipose tissue passes through the metal blades between the two injectors. However, the kinetic energy generated at this time affects the polarity of the cells. We believe that in IPs 1 and 2, this electrical polarity affects the relationship between saline and stromal cells and helps to separate stromal cells more successfully. However, this is just speculation, further research is required to prove this theory. In this research, we call the final product total stromal cells (TOST). Since mechanically obtained stromal cells can be used in a wide range of indications, the proposed IP system will help surgeons isolate the desired TOST with our new technique, MEST, using sharp blades to avoid excessive blunt pressure, the isolation of stromal cells by cutting fat prevents the death of fat tissue and stromal cells and allows high viability and cell counts.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: H Eray Copcu https://orcid.org/0000-0003-3799-3504
How to cite this article
Eray Copcu, H. Indication-based protocols with different solutions for mechanical stromal-cell transfer. Scars, Burns & Healing, Volume 7, 2021. DOI: 10.1177/20595131211047830
References
- 1.Schildberg FA, Donneberg VS S. Stromal cells in health and disease. Cytometry 2018; 93: 871–875. [DOI] [PubMed] [Google Scholar]
- 2.Alexander RW. Understanding mechanical emulsification (nanofat) versus enzymatic isolation of tissue stromal vascular fraction (tSVF) cells from adipose tissue: potential uses in biocellular regenerative medicine. J Prolother 2016; 8: 947–960. [Google Scholar]
- 3.Guo J, Widgerow AD, Banyard D, et al. Strategic sequences in fat graft survival. Ann Plast Surg 2015; 74: 376–382. [DOI] [PubMed] [Google Scholar]
- 4.Tonnard P, Verpaele A, Peeters G, et al. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg 2013; 132: 1017–1026. [DOI] [PubMed] [Google Scholar]
- 5.Morestin H. Quelques cas de greffes graisseuse appliquees aIa chirurgie reparatrice. Bull Mem Soc Chir (Paris) 1915; 41: 1631. [Google Scholar]
- 6.Jarrell BE, Williams SK, Stokes G, et al. Use of freshly isolated capillary endothelial cells for the immediate establishment of a monolayer on a vascular graft at surgery. Surgery 1986; 100: 392–399. [PubMed] [Google Scholar]
- 7.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7: 211–228. [DOI] [PubMed] [Google Scholar]
- 8.Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015; 4: 713–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friji MT. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg 2014; 134: 333e–334e. [DOI] [PubMed] [Google Scholar]
- 10.Bernardini FP, Gennai A, Izzo L, et al. Superficial enhanced fluid Fat injection (SEFFI) to correct volume defects and skin aging of the face and periocular region. Aesthetic Surg J 2015; 35: 504–515. [DOI] [PubMed] [Google Scholar]
- 11.Yao Y, Cai J, Zhang P, et al. Adipose stromal vascular fraction Gel grafting: a New method for tissue volumization and rejuvenation. Dermatol Surg 2018; 44: 1278–1286. [DOI] [PubMed] [Google Scholar]
- 12.Condé-Green A, Kotamarti VS, Sherman LS, et al. Shift toward mechanical isolation of adipose-derived stromal vascular fraction: review of upcoming techniques. Plast Reconstr Surg Glob Open 2016; 4: 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernardini FP, Gennai A. Fluid fat injection for volume restoration and skin regeneration of the periocular aesthetic unit. JAMA Facial Plast Surg 2016; 18: 68–70 [DOI] [PubMed] [Google Scholar]
- 14.van Dongen JA, Stevens HP, Harmsen MC, et al. Mechanical micronization of lipoaspirates: squeeze and emulsification techniques. Plast Reconstr Surg 2017; 139: 1369–170e. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SR, Hewett S, Ross L, et al. Regenerative cells For facial surgery: biofilling and biocontouring. Aesthetic Surg J 2017; 37(Suppl_3): S16–S32. [DOI] [PubMed] [Google Scholar]
- 16.Rohrich RJ, Mahedia M, Shah N, et al. Role of fractionated fat in blending the lid-cheek junction. Plast Reconstr Surg 2018; 142: 56–65. [DOI] [PubMed] [Google Scholar]
- 17.Glass GE, Ferretti P. Adipose-derived stem cells in aesthetic surgery. Aesthetic Surg J 2019; 39: 423–438. [DOI] [PubMed] [Google Scholar]
- 18.Verpaele A, Tonnard P. Discussion: nanofat cell aggregates: a nearly constitutive stromal cell inoculum for regenerative site-specific therapies. Plast Reconstr Surg 2019; 144: 1089–1090. [DOI] [PubMed] [Google Scholar]
- 19.https://en.wikipedia.org/wiki/Nanotechnology.
- 20.Stuzin JM. Discussion: nanofat grafting: basic research and clinical applications. Plast Reconstr Surg 2013; 132: 1027–1028. [DOI] [PubMed] [Google Scholar]
- 21.van Dongen JA, Tuin AJ, Spiekman M, et al. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: a systematic review. J Tissue Eng Regen Med 2018; 12: 261–274. [DOI] [PubMed] [Google Scholar]
- 22.Sesé B, Sanmartín JM, Ortega B, et al. Nanofat cell aggregates: a nearly constitutive stromal cell inoculum for regenerative site-specific therapies. Plast Reconstr Surg 2019; 144: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banyard DA, Sarantopoulos CN, Borovikova AA, et al. Phenotypic analysis of stromal vascular fraction after mechanical shear reveals stress-induced progenitor populations. Plast Reconstr Surg 2016; 138: 237–47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohrich RJ, Wan D. Making sense of stem cells and fat grafting in plastic surgery: the hype, evidence, and evolving U.S. Food and drug administration regulations. Plast Reconstr Surg 2019; 143: 417–424. [DOI] [PubMed] [Google Scholar]
- 25.He Y, Xia J, Chen H, et al. Human adipose liquid extract induces angiogenesis and adipogenesis: a novel cell-free therapeutic agent. Stem Cell Res Ther 2019; 10: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tremolada C, Colombo V, Ventura C. Adipose tissue and mesenchymal stem cells: state of the art and Lipogems® technology development. Curr Stem Cell Rep 2016; 2: 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen SR, Womack H, Ghanem A. Fat grafting for facial rejuvenation through injectable tissue replacement and regeneration: a differential, standardized, anatomic approach. Clin Plast Surg 2020; 47: 31–41. [DOI] [PubMed] [Google Scholar]
- 28.Trivisonno A, Alexander RW, Baldari S, et al. Intraoperative strategies for minimal manipulation of autologous adipose tissue for cell- and tissue-based therapies: concise review. Stem Cells Transl Med 2019; 8: 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen SR, Womack H. Injectable tissue replacement and regeneration: anatomic fat grafting to restore decayed facial tissues. Plast Reconstr Surg Glob Open 2019; 7: 2293–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsekouras A, Mantas D, Tsilimigras I, et al. Comparison of the viability and yield of adipose-derived stem cells (ASCs) from different donor areas. In vivo (Athens, Greece) 2017; 31: 1229–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiryaki K. Mekanik ve enzimatik yöntem ile izole edilen stromal vasküler fraksiyonun yara iyileşmesi üzerine etkisisnin in vitro incelenmesi. J Istanb Fac Med 2020; 1: 28–29. [Google Scholar]
- 32.Cohen SR, Tiryaki T, Womack HA, et al. Cellular optimization of nanofat: comparison of two nanofat processing devices in terms of cell count and viability. Aesthetic Surg J Open Forum 2019; 1: ojz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zocchi M, Vindigni V, Pagani A, et al. Regulatory, ethical, and technical considerations on regenerative technologies and adipose-derived mesenchymal stem cells. Eur J Plast Surg 2019; 42: 531–548. [Google Scholar]
- 34.Ghiasloo M, Lobato RC, Díaz JM, et al. Expanding clinical indications of mechanically isolated stromal vascular fraction: a systematic review. Aesthet Surg J 2020; 40: NP546–NP560. [DOI] [PubMed] [Google Scholar]
- 35.Jones IA, Wilson M, Togashi R, et al. Jr. A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: a study protocol. BMC Musculoskelet Disord 2018; 19: 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lander EB, Berman MH, See JR. Stromal vascular fraction combined with shock wave for the treatment of Peyronie’s disease. Plast Reconstr Surg Glob Open 2016; 4: e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haahr MK, Jensen CH, Toyserkani NM, et al. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: an open-label phase I clinical trial. EBioMedicine 2016; 5: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.http://sphweb.bumc.bu.edu/otlt/MPHModules/PH/PH709_BasicCellBiology/PH709_BasicCellBIology4.html.
- 39.Khlusov IA, Dekhtyar Y, Sharkeev YP, et al. Nanoscale electrical potential and roughness of a calcium phosphate surface promotes the osteogenic phenotype of stromal cells. Materials (Basel) 2018; 11: 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.https://www.usgs.gov/media/images/water-molecules-and-their-interaction-salt-molecules.