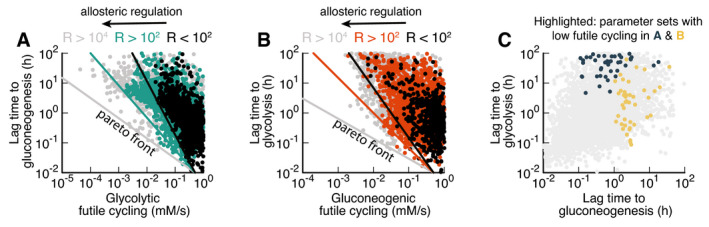
A, BModel calculated for randomized sets of protein abundancies, reaction rates, Michaelis constants, allosteric interactions, transcriptional regulation, see Appendix. Each point corresponds to a parameter set that allows exponential growth on both glycolytic and gluconeogenic carbons, as well switching between both conditions. Data are colored according to the total regulation R, i.e., the sum of fold changes in enzyme activities between glycolysis and gluconeogenesis, , where and are protein abundances in glycolysis and gluconeogenesis of protein i and the strength of the allosteric regulation. For standard E. coli parameters R = 23. R > 104 are likely unphysiological. Lines indicate Pareto front and are drawn by hand.