Abstract
Background
The changes in shield strategies, treatments, emergence variants, and healthcare pathways might shift the profile and outcome of patients hospitalized with COVID-19 in successive waves of the outbreak.
Methods
We retrospectively analysed the characteristics and in-hospital outcomes of all patients admitted with COVID-19 in eight university hospitals of Catalonia (North-East Spain) between Feb 28, 2020 and Feb 28, 2021. Using a 7-joinpoint regression analysis, we split admissions into four waves. The main hospital outcomes included 30-day mortality and admission to intensive care unit (ICU).
Findings
The analysis included 17,027 subjects admitted during the first wave (6800; 39.9%), summer wave (1807; 10.6%), second wave (3804; 22.3%), and third wave (4616; 27.1%). The highest 30-day mortality rate was reported during the first wave (17%) and decreased afterwards, remaining stable at 13% in the second and third waves (overall 30% reduction); the lowest mortality was reported during the summer wave (8%, 50% reduction). ICU admission became progressively more frequent during successive waves. In Cox regression analysis, the main factors contributing to differences in 30-day mortality were the epidemic wave, followed by gender, age, diabetes, chronic kidney disease, and neoplasms.
Interpretation
Although in-hospital COVID-19 mortality remains high, it decreased substantially after the first wave and is highly dependent of patient's characteristics and ICU availability. Highest mortality reductions occurred during a wave characterized by younger individuals, an increasingly frequent scenario as vaccination campaigns progress.
Funding
This work did not receive specific funding.
Keywords: Coronavirus disease 2019 (Covid-19), Hospital mortality, Clinical characteristics, Socioeconomic characteristics, Risk factors
Introduction
Soon after the first outbreak of coronavirus disease 2019 (COVID-19) in Italy on late February 2020, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic spread rapidly across all European countries, leading to more than two million cases and 100,000 deaths in three months in this continent [1]. Social distancing measures—including strict lockdowns—dictated in many European countries successfully reduced the average reproduction number (R-value) below 1.0 by March 2020 [2], which was followed by a rapid reduction in SARS-CoV-2 incident cases. However, countries worldwide have experienced successive waves of the pandemic in an scenario of alternate intensification and lifting of restrictions [3,4]. Vaccination campaigns are expected to minimize the risk of future waves and change the characteristics of hospitalized patients. However, the capacity of vaccines for achieving long-term immunity and protect against future strains is uncertain [5], and various authors have warned on the risk of further waves despite vaccination if restrictions are lifted inadequately [6].
During the first wave (i.e., sharp incidence increase) of COVID-19, various retrospective studies of hospitalized patients provided a global picture of the presenting characteristics of these patients [[7], [8], [9]], as well as the determinants of severity and death [[10], [11], [12], [13], [14]]. Although highly heterogeneous, these reports identified several clinical characteristics consistently associated with severe illness or death. Since then, the management and therapeutic landscape of COVID-19 have evolved and new SARS-CoV-2 variants potentially associated with different disease severity and transmission have emerged, with limited information regarding the prevalence and dynamics of circulating genomes worldwide [[15], [16], [17]]. Furthermore, changes in incidence due to shielding and physical distancing interventions [18] and efforts to deploy strategies for early identification of cases is reshaping the healthcare pathways of COVID-19 patients in many countries.
Changes in shield strategies, treatment, emerging SARS-CoV-2 variants, and healthcare pathways are expected to shift the profile of patients hospitalized with COVID-19 throughout successive waves. Nevertheless, the way the pandemic has evolved throughout the various waves differs between countries in the onset and severity pattern [[19], [20], [21], [22], [23]]. In Spain, the first case of COVID-19 was reported in late February [24] and elicited one of the major outbreaks in incidence globally and a national lockdown that lasted for 98 days [1]. The reduction in daily new COVID-19 cases below 1000 (0.2 per 100,000 inhabitants) by April 25 [25] led to a “new normal” scenario in which healthcare authorities tried to contain the SARS-CoV-2 spread in a trade-off between banning of social, cultural, and commercial activities and flattening the epidemic curve. Since then, the country has experienced three additional waves, according to the ministry of health [24]. Although this pattern of alternate periods of high incidence (i.e., waves) has been experienced in many countries worldwide, there is little information regarding the changes in patient profile and hospital outcomes throughout waves in large cohorts. In this study, we investigated the presenting characteristics and outcomes of patients hospitalized during the successive waves (from Feb 2020 to Feb 2021) of the COVID-19 outbreak in Catalonia, a region with 7.5 million inhabitants that was among the two leading regions in incidence in Spain.
Methods
Study design and setting
This was a retrospective analysis of patients hospitalized due to severe COVID-19 in eight university hospitals of Catalonia between Feb 28, 2020 and Feb 28, 2021. The end of the observation period was established on Apr 20, 2021. The hospitals belong to the Institut Català de la Salut (Catalan Institute of Health), a major healthcare provider that accounts for 30% of the total acute hospitalizations in the region and has a strong territorial representation. Catalonia is an industry- and services-driven economy country with 7.5 million inhabitants, 5.2 of which live in the 2268 Km2 of the Urban Region of Barcelona. Outbreak waves were formally defined using a 7-joinpoint regression analysis [26] of the distribution of hospital admissions reported between the investigated period. The analysis depicted four waves, three of them with a clear pattern of hospital admissions increase and decrease and one of them (the “summer wave”) remaining below 33 daily admissions over almost four months (Fig. 1). The alpha variant became dominant from July 2020 (Fig. S1, Supplementary File 1).
Fig. 1.
Distribution of hospital admissions due to COVID-19 across the analysed period (Feb 28, 2020 to Feb 28, 2021). The 7-joinpoint regression analysis revealed the presence of four waves, one of them below 33 daily admissions.
Patients were identified from the central electronic hospital record of the Catalan Institute of Health, which systematically collects routine hospitalization data —including the diagnosis associated with admission—, resource use during hospital stay, and pharmacy information. The study included all patients of any age admitted to the hospital with a COVID-19 diagnosis. Data regarding existing comorbidities, sociodemographic characteristics, and health behaviour were gathered from the Information System for the Development of Primary Care Research [27], which is linked to the electronic hospital record through a unique patient identification number. All data were handled according to the General Data Protection Regulation 2016/679 on data protection and privacy for all individuals within the European Union and the local regulatory framework regarding data protection. The study protocol was approved by the independent ethics committee of the Hospital Germans Trias i Pujol (Badalona, Spain), which waived obtaining informed consent.
Study variables and outcomes
The primary objective of the study was to describe the presenting features and 30-day mortality of COVID-19 patients admitted within each of the outbreak waves. Secondary objectives included describing the frequency of severe clinical events during the hospital stay and investigate the influence of the presenting features on hospital outcomes associated with critical illness and mortality throughout the successive waves.
The presenting features of hospitalized COVID-19 patients were described based on sociodemographic and clinical data at admission. Sociodemographic characteristics included age, gender, smoking status, and socioeconomic status, defined based on the MEDEA index, an ecological measure of deprivation at the district (i.e., residential areas within the municipality) level [28,29]. The MEDEA index allows stratifying each individual according to five mutually-exclusive levels of rurality and deprivation (i.e., lack of access to social and material resources) of the district: rural, semi-rural, semi-urban, and urban areas, the last grouped into low, moderate, high, and very high deprivation level. Clinical characteristics included the presence of the following relevant comorbidities: hypertension, cardiovascular disease (i.e., stroke, heart disease, ischemic heart disease, or atrial fibrillation), type 1 or 2 diabetes mellitus, chronic kidney disease, liver disease (i.e., chronic liver disease or viral hepatitis), respiratory disease (i.e., asthma, chronic bronchitis, or chronic obstructive pulmonary disease), immunosuppression (i.e., HIV or history of solid organ transplant), and neoplasia. The comorbidity burden was described using the Charlson comorbidity index, and the overall patient complexity was stratified using the adjusted morbidity groups (GMA, Grups de Morbiditat Ajustada, i.e, Morbidity-Adjusted Groups), a population-based tool for health-risk assessment described previously [30,31]. In brief, the GMA tool considers the type of disease—acute or chronic—, number of systems affected, and complexity of each disease for grouping people in four health-risk categories: 1) Initial risk (healthy stage, including GMA scores up to the 50th percentile of the total population); 2) low risk, 50th to 80th percentiles; 3) moderate risk, 80th to 95th percentiles, and 4) high risk, above the 95th percentile. We also collected data regarding laboratory tests performed up to seven days after hospital admission, except for glomerular filtration rate [GFR] and glycated hemoglobin, which corresponded to the last laboratory assessment preceding admission; the smoking status, and the body mass index (BMI), grouped according to the CDC criteria for weight stratification [32].
Additional hospital outcomes aside from death within 30 days after hospital admission included in-hospital death and the following severe clinical events: the need for advanced respiratory support (i.e., non-invasive mechanical ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation [ECMO]), and transfer to an ICU. Transfer to special units designated for treating COVID-19 patients with advanced care needs (i.e., including general, paediatric, and new-born intensive care units, intermediate care units, coronary unit, burn unit, stroke unit, and heart surgery) were also considered ICU transfers. We also collected other hospital outcomes such as the length of hospital stay and destination at discharge (i.e., home, skilled nursing home). Admissions that occurred earlier than seven days after the discharge of index admission (i.e., first time admitted to hospital because of COVID-19) were considered a single episode, and data regarding the two admissions were pooled together. Finally, we used billing information of the included hospitals to retrieve quantitative data on the overall prescription of drugs used for treating patients with COVID-19 during year 2020, using 2019 data as reference.
Statistical analysis
Continuous variables were described as the mean and standard deviation (SD) or median and interquartile range (IQR, defined by the 25th and 75th percentiles) and categorical variables as number and percentage over available data. The model fit for the 7-joinpoint regression analysis was performed using a Grid Search method and assuming a constant variance (homocedasticity) and an uncorrelated errors model; the annual percent change estimates resulting from the model are summarized in Table S1 (Supplementary appendix). Survival was described using Kaplan-Meier estimates and compared with the Log-rank test. For each event, the follow-up time was defined between hospitalization and the event. Patients were followed until censored (i.e., discharge, lost to follow-up, or end of the observation period). The influence of patients' characteristics at admission on the risk of main hospitalization outcomes (i.e., 30-day and in-hospital death) was investigated using a Cox proportional-hazard model adjusted for variables of interest. Missing values were imputed using a multiple imputation analysis by chained equations with 10 imputed datasets; the percentage of missing values are listed in Table S2. Proportional hazards assumption was assessed according to the scaled Schoenfeld residuals [33]. The model was computed for the entire study sample (i.e., patients admitted at any time within the investigated period) and for each wave separately. A similar analysis was performed for transfer to a special unit. Results of the models were presented as the hazard ratio (HR) and the 95% confidence interval (CI). Owing to the limited linearity of the hazard functions for age, this variable was included as a 5-knot restricted cubic spline [34]. Finally, the estimated cumulative incidence function for both 30-day and in-hospital mortality assuming a competing risk scenario was computed, considering ICU transfer as the competing risk event and home discharge as no event or censoring. The significance threshold was set at a two-sided alpha value of 0.05 for all analyses. All analyses were performed using the R statistical package (version 4.0.2 or higher) and plotted using ggplot2 and Survminer.
Results
Patient characteristics
Between Feb 28, 2020 and Feb 28, 2021, 17,027 patients were admitted to hospitals of the Catalan Institute of Health due to COVID-19: 6802 (29.9%) before Jun 6, 2020 (first wave), 1807 (10.6%) between Jun 7 and Sep 22, 2020 (summer wave), 3807 (22.3%) between Sept 23 and Dec 12, 2020 (second wave), and 4616 (27.1%) between Dec 13, 2020 and Feb 28, 2021 (third wave). By the time of the database closure (i.e., March 22, 2021), 108 patients had not yet been discharged. Table 1 summarizes the main demographic and clinical characteristics of patients hospitalized during the investigated period. Patients admitted within the first, second, and third waves had similar demographic characteristics, although those in the third wave were older. The summer wave was characterized by a higher proportion of patients with low health risk (i.e., lower GMA level) and younger. Hypertension, diabetes, and cardiovascular disease were the leading comorbidities in the overall study sample and each of the investigated periods. The comparison of comorbidities revealed significant differences between waves regarding the proportion of patients with diabetes, hypertension, cardiovascular disease, and chronic kidney disease at the time of admission. Overall, the proportion of patients with Charlson score ≥ 3 increased in the second and third waves. The percentage of individuals with obesity on admission increased in the third wave. The proportion of patients in the lowest socioeconomic level increased after the first wave.
Table 1.
Presenting characteristics of patients admitted to hospital due to Covid-19.
| No. | Overall (N = 17,027) |
First wave (N = 6800) |
Summer wave (N = 1807) |
Second wave (N = 3804) |
Third wave (N = 4616) |
|
|---|---|---|---|---|---|---|
| Sociodemographic characteristics | ||||||
| Sex (female), n (%) | 17,027 | 7239 (42.5%) | 2929 (43.1%) | 782 (43.3%) | 1623 (42.7%) | 1905 (41.3%) |
| Age (years), median (IQR) | 16,989 | 64.0 (51.0;76.0) | 64.0 (51.0;75.0) | 59.0 (44.0;73.0) | 64.0 (50.0;76.0) | 66.0 (54.0;77.0) |
| Age group, n (%) | ||||||
| 0–20 | 277 (1.63%) | 62 (0.91%) | 55 (3.04%) | 90 (2.37%) | 70 (1.53%) | |
| 20–30 | 564 (3.32%) | 198 (2.91%) | 98 (5.42%) | 142 (3.74%) | 126 (2.75%) | |
| 30–40 | 1096 (6.45%) | 409 (6.01%) | 192 (10.6%) | 266 (7.00%) | 229 (5.00%) | |
| 40–50 | 1959 (11.5%) | 837 (12.3%) | 257 (14.2%) | 414 (10.9%) | 451 (9.84%) | |
| 50–60 | 2975 (17.5%) | 1272 (18.7%) | 311 (17.2%) | 650 (17.1%) | 742 (16.2%) | |
| 60–70 | 3422 (20.1%) | 1346 (19.8%) | 325 (18.0%) | 783 (20.6%) | 968 (21.1%) | |
| 70–80 | 3718 (21.9%) | 1552 (22.8%) | 302 (16.7%) | 779 (20.5%) | 1085 (23.7%) | |
| 80–90 | 2392 (14.1%) | 917 (13.5%) | 198 (11.0%) | 523 (13.8%) | 754 (16.4%) | |
| 90–109 | 586 (3.45%) | 207 (3.04%) | 69 (3.82%) | 151 (3.98%) | 159 (3.47%) | |
| District deprivation levela | 15,741 | |||||
| Rural | 563 (3.58%) | 122 (1.92%) | 73 (4.50%) | 154 (4.40%) | 214 (5.02%) | |
| Semi-rural | 1060 (6.73%) | 261 (4.10%) | 200 (12.3%) | 261 (7.46%) | 338 (7.93%) | |
| Semi-urban | 1435 (9.12%) | 370 (5.82%) | 123 (7.59%) | 418 (11.9%) | 524 (12.3%) | |
| Urban low deprivation | 2316 (14.7%) | 982 (15.4%) | 190 (11.7%) | 493 (14.1%) | 651 (15.3%) | |
| Urban moderate deprivation | 2327 (14.8%) | 1006 (15.8%) | 243 (15.0%) | 483 (13.8%) | 595 (14.0%) | |
| Urban high deprivation | 4020 (25.5%) | 1849 (29.1%) | 395 (24.4%) | 771 (22.0%) | 1005 (23.6%) | |
| Urban very high deprivation | 4020 (25.5%) | 1770 (27.8%) | 397 (24.5%) | 920 (26.3%) | 933 (21.9%) | |
| Clinical characteristics | 16,140 | |||||
| Health risk (GMA level), n (%) | ||||||
| Initial risk (health stage) | 1862 (11.5%) | 699 (10.8%) | 279 (16.6%) | 452 (12.5%) | 432 (9.88%) | |
| Low risk | 4116 (25.5%) | 1719 (26.5%) | 442 (26.4%) | 897 (24.9%) | 1058 (24.2%) | |
| Moderate risk | 5721 (35.4%) | 2378 (36.7%) | 531 (31.7%) | 1241 (34.4%) | 1571 (35.9%) | |
| High risk | 4441 (27.5%) | 1690 (26.1%) | 425 (25.3%) | 1014 (28.1%) | 1312 (30.0%) | |
| Charlson comorbidity index, median (IQR) | 15,192 | 1.00 (0.00;2.00) | 1.00 (0.00;2.00) | 1.00 (0.00;2.00) | 1.00 (0.00;2.00) | 1.00 (0.00;2.00) |
| Chalson comorbidity index, n (%) | ||||||
| 0–2 | 9657 (63.6%) | 3975 (64.7%) | 1067 (67.4%) | 2132 (62.9%) | 2483 (60.9%) | |
| 2 | 2242 (14.8%) | 886 (14.4%) | 211 (13.3%) | 510 (15.0%) | 635 (15.6%) | |
| 3–13 | 3293 (21.7%) | 1278 (20.8%) | 305 (19.3%) | 749 (22.1%) | 961 (23.6%) | |
| Specific comorbiditiesb, n (%) | 16,758 | |||||
| Diabetes | 4156 (24.8%) | 1504 (22.4%) | 432 (24.7%) | 952 (25.4%) | 1268 (27.9%) | |
| Liver diseasec | 1678 (10.0%) | 674 (10.0%) | 151 (8.64%) | 374 (9.98%) | 479 (10.5%) | |
| Hypertension | 7600 (45.4%) | 2985 (44.4%) | 713 (40.8%) | 1721 (45.9%) | 2181 (48.0%) | |
| Immunosuppressiond | 80 (0.48%) | 33 (0.49%) | 14 (0.80%) | 17 (0.45%) | 16 (0.35%) | |
| Cardiovascular diseasee | 3677 (21.9%) | 1431 (21.3%) | 335 (19.2%) | 844 (22.5%) | 1067 (23.5%) | |
| Chronic kidney disease | 2390 (14.3%) | 876 (13.0%) | 210 (12.0%) | 578 (15.4%) | 726 (16.0%) | |
| Neoplasia | 2598 (15.5%) | 1003 (14.9%) | 236 (13.5%) | 598 (16.0%) | 761 (16.8%) | |
| Chronic respiratory diseasef | 2342 (14.0%) | 926 (13.8%) | 226 (12.9%) | 509 (13.6%) | 681 (15.0%) | |
| Smoking status, n (%) | 13,466 | |||||
| Never smoker | 7632 (56.7%) | 3131 (57.1%) | 804 (59.2%) | 1687 (56.6%) | 2010 (55.1%) | |
| Active smoker | 1068 (7.93%) | 398 (7.26%) | 124 (9.12%) | 234 (7.85%) | 312 (8.56%) | |
| Former smoker | 4766 (35.4%) | 1950 (35.6%) | 431 (31.7%) | 1061 (35.6%) | 1324 (36.3%) | |
| Body mass index (Kg/m2), median (IQR) | 8944 | 29.3 (26.0;33.0) | 29.1 (25.9;32.5) | 29.0 (25.7;33.2) | 29.1 (25.9;33.1) | 29.7 (26.3;33.3) |
| Body mass index categoriesg | ||||||
| Underweight | 152 (1.70%) | 47 (1.29%) | 28 (2.96%) | 40 (2.03%) | 37 (1.56%) | |
| Normal | 1488 (16.6%) | 617 (16.9%) | 160 (16.9%) | 347 (17.6%) | 364 (15.3%) | |
| Overweight | 3353 (37.5%) | 1440 (39.5%) | 348 (36.8%) | 718 (36.4%) | 847 (35.7%) | |
| Obese | 3951 (44.2%) | 1544 (42.3%) | 410 (43.3%) | 870 (44.1%) | 1127 (47.5%) | |
| Laboratory assessments | ||||||
| Glycated hemoglobin (%), median (IQR) | 6790 | 6.10 (5.60;7.10) | 6.10 (5.60;7.00) | 6.10 (5.60;7.10) | 6.10 (5.60;7.20) | 6.20 (5.70;7.20) |
| Glomerular filtration rateh (mL/min/1.73m2), median (IQR) | 13,706 | |||||
| severely decreased | 750 (5.47%) | 261 (4.74%) | 76 (5.12%) | 177 (5.85%) | 236 (6.39%) | |
| moderately decreased | 2155 (15.7%) | 801 (14.5%) | 214 (14.4%) | 469 (15.5%) | 671 (18.2%) | |
| mildly decreased | 4767 (34.8%) | 2026 (36.8%) | 466 (31.4%) | 1019 (33.7%) | 1256 (34.0%) | |
| normal or high | 6034 (44.0%) | 2419 (43.9%) | 727 (49.0%) | 1359 (44.9%) | 1529 (41.4%) | |
| Albumin (g/L), median (IQR) | 6795 | 34.0 (30.0;37.5) | 33.0 (29.6;36.5) | 35.0 (31.0;38.5) | 34.6 (30.5;38.0) | 34.0 (30.5;37.5) |
| D-dimer (ng/mL), median (IQR) | 5926 | 555 (316;1335) | 545 (311;1461) | 544 (314;1174) | 560 (319;1300) | 573 (325;1281) |
| Ferritin (ng/mL), median (IQR) | 6527 | 691 (346;1334) | 770 (378;1475) | 571 (284;1169) | 658 (323;1245) | 667 (350;1277) |
| Interleukin-6 (pg/mL), median (IQR) 2513 | 28.1 [10.5;83.4] | 50.9 (15.9;276) | 22.7 (10.2;76.6) | 21.1 (8.30;50.3) | 20.2 (8.52;45.6) | |
| Lymphocite count (x109/L), median (IQR) 9000 | 1.10 [0.75;1.55] | 1.09 (0.74;1.50) | 1.20 (0.86;1.67) | 1.13 (0.78;1.60) | 1.08 (0.74;1.53) | |
| C-reactive protein (mg/dL), median (IQR) | 8051 | 64.9 (28.7;119) | 75.8 (31.6;139) | 50.6 (25.6;103) | 58.6 (27.4;108) | 60.5 (28.0;108) |
GMA: Adjusted morbidity groups. IQR: interquartile range (25th and 75th percentiles). SD: standard deviation.
arural (<7500 inhab and < 100 inhab/Km2), semi-rural (<7500 and > 100 inhab/Km2 or > 7500 inhab and < 100 inhab/Km2), semi-urban (7500–10,000 inhab and 100–150 inhab/Km2), urban (>10,000 inhab and > 150 inhab/Km2); deprivation levels of urban areas correspond nation-level quartiles.
bCategories are not mutually exclusive. cChronic liver disease or viral hepatitis. dHIV infection or history of solid organ transplant. estroke, heart disease, ischemic heart disease, or atrial fibrillation. fasthma, chronic bronchitis, or chronic obstructive pulmonary disease. gstratified according to the CDC criteria: underweight (< 18.5), normal (18.5 to <25), overweight (25.0 to <30), and obese (≥ 30). hseverely decreased (〈30), moderately decreased (30–60), mildly decreased (61–90), normal or high (> 90).
Hospital outcomes
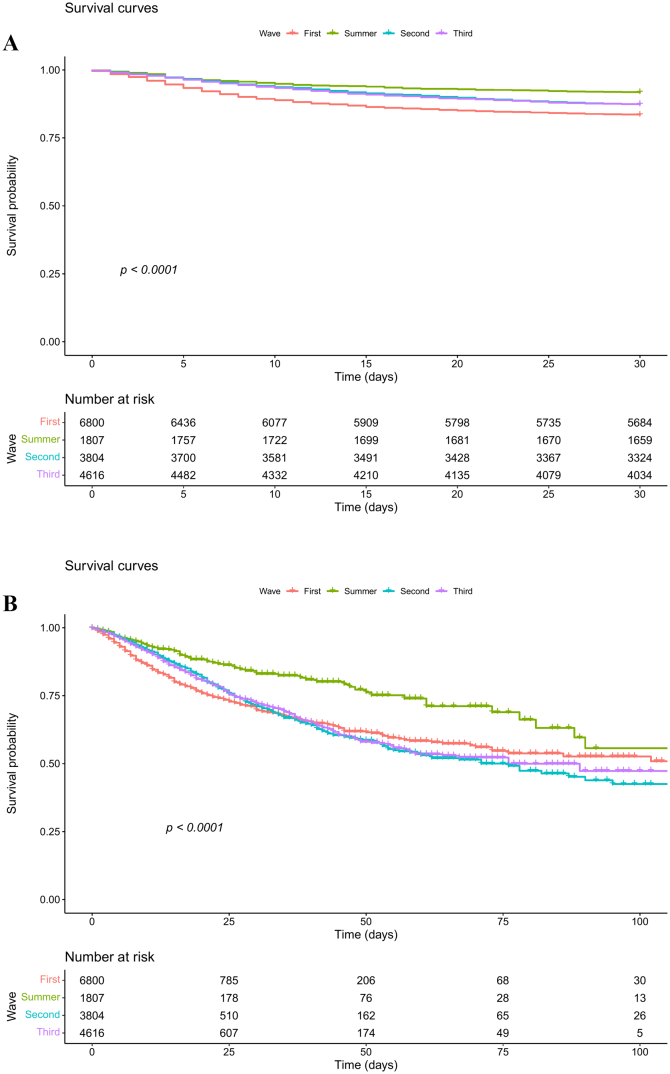
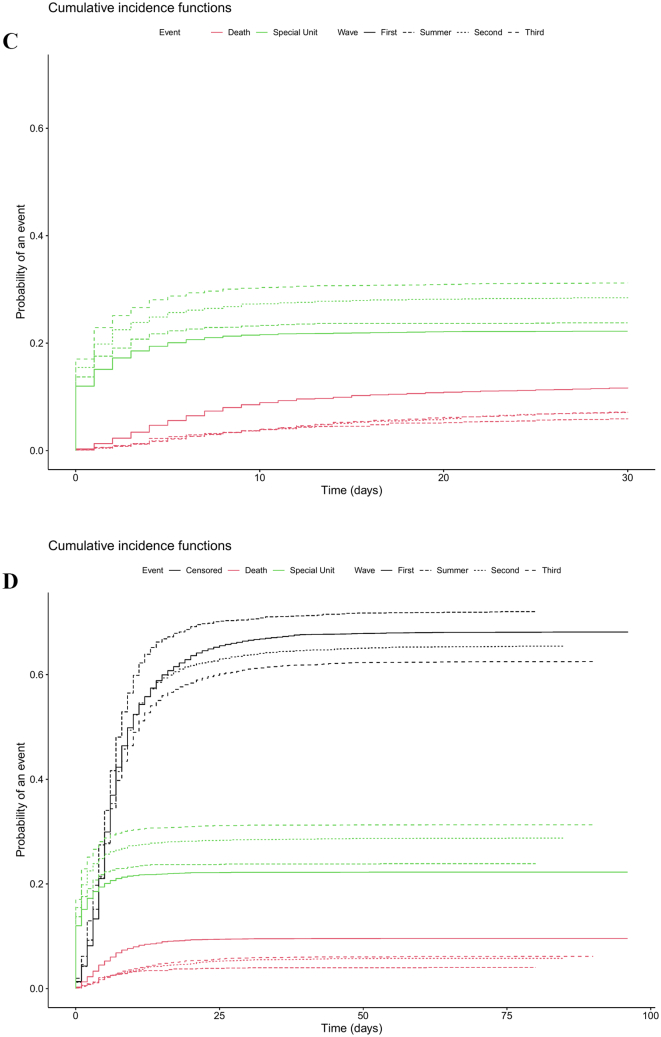
The 30-day mortality rate was highest in the first wave (17%) and decreased afterwards, remaining stable at 13% in the second and third waves; the lowest mortality was reported during the summer wave (8.30%) (Table 2). Likewise, survival was significantly higher among patients hospitalized during the summer wave; this trend was also observed in the competing risk analysis (Fig. 2). On the other hand, ICU transfers progressively increased throughout the analysed period, from 22% in the first wave to 31% in the third wave (Table 2). The competing risks analysis showed that patients admitted during the first wave had the lowest probability of being transferred to an ICU and the highest probability of dying (Fig. 2). The median (IQR) follow-up time for 30-day mortality, in-hospital mortality, and time to ICU transfer were 30.0 (30.0; 30.0), 7.00 (4.00; 14.0), and 5.00 (2.00; 8.00), respectively.
Table 2.
Hospitalization outcomes and management of the Covid-19 episode.
| Overall (N = 17,027) |
First wave (N = 6800) |
Summer wave (N = 1807) |
Second wave (N = 3804) |
Third wave (N = 4616) |
|
|---|---|---|---|---|---|
| Hospital outcomes (N = 17,027) | |||||
| 30-day mortality a, n (%) | 2356 (13.8%) | 1132 (16.6%) | 150 (8.30%) | 486 (12.8%) | 588 (12.7%) |
| In-hospital mortality, n (%) | 2228 (13.1%) | 1027 (15.1%) | 132 (7.30%) | 480 (12.6%) | 589 (12.8%) |
| Transfer to a ICU, n (%) | 4484 (26.3%) | 1514 (22.3%) | 431 (23.9%) | 1094 (28.8%) | 1445 (31.3%) |
| Length of hospital stay (days), median (IQR) | 7.00 (4.00;14.0) | 7.00 (4.00;14.0) | 7.00 (4.00;12.0) | 8.00 (4.00;14.0) | 8.00 (4.00;15.0) |
| Destination at discharge b, n (%) | |||||
| Home | 10,192 (60.0%) | 3534 (52.0%) | 1189 (65.8%) | 2490 (65.6%) | 2979 (65.0%) |
| Skilled nursing facility for intermediate care | 1630 (9.60%) | 650 (9.56%) | 153 (8.47%) | 356 (9.37%) | 471 (10.3%) |
| Management features (N = 16,953) | |||||
| Need of respiratory support, n (%) | 9264 (54.6%) | 3791 (55.8%) | 773 (42.8%) | 2093 (55.2%) | 2607 (57.3%) |
| Type of respiratory support c, n (%) | |||||
| Oxygen therapy | 7160 (42.2%) | 3006 (44.2%) | 585 (32.4%) | 1597 (42.1%) | 1972 (43.3%) |
| non-invasive positive pressure | 2495 (14.7%) | 759 (11.2%) | 233 (12.9%) | 627 (16.5%) | 876 (19.2%) |
| Invasive mechanical ventilation | 1697 (10.0%) | 759 (11.2%) | 134 (7.42%) | 366 (9.64%) | 438 (9.62%) |
| Extracorporeal membrane oxygenation | 117 (0.69%) | 52 (0.76%) | 11 (0.61%) | 31 (0.82%) | 23 (0.51%) |
ICU: intensive care unit. IQR: interquartile range (25th and 75th percentiles).
15.5% of 30-day deaths occurred post-discharge; range: 12.8% (third wave) to 26.0% (summer wave). Patients still at hospital after 30 days accounted for 9.3% in the overall study sample; range: 8.8% (summer wave) to 10.0% (second wave).
Only the two most frequent destinations are presented. cThe same patient could receive more than one type of respiratory support during hospital stay.
Fig. 2.
Survival curve (Kaplan-Meier estimate) of patients admitted to hospital because of COVID-19 during the first wave (Feb 28 to Jun 6, red line), summer wave (Jun 7 to Sep 22, green line), second wave (Sep 23 to Dec 12, blue line), and third wave (Dec 13, 2020 to Feb 28, 2021) of the COVID-19 outbreak. A: In-hospital survival. B: 30-day survival. The p-value corresponds to the Log-rank test for survival differences between the two curves. C: competing risk analysis for 30-day mortality. D: competing risk analysis for in-hospital mortality. The censoring proportion for 30-day and in-hospital survival were 86.2% and 86.9%, all due to end of follow-up. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
During the first wave, we observed a remarkable increase in the expenditure of drugs intended for treating COVID-19 like chloroquine, hydroxychloroquine, lopinavir/ritonavir, and tocilizumab (Fig. S2, Supplementary File 1). No purchases of these drugs were reported after May 2020, except for tocilizumab, which consumption was maintained throughout successive waves. Study hospitals started using remdesivir in July 2020 and maintained its use throughout the investigated period.
Determinants of hospital outcomes
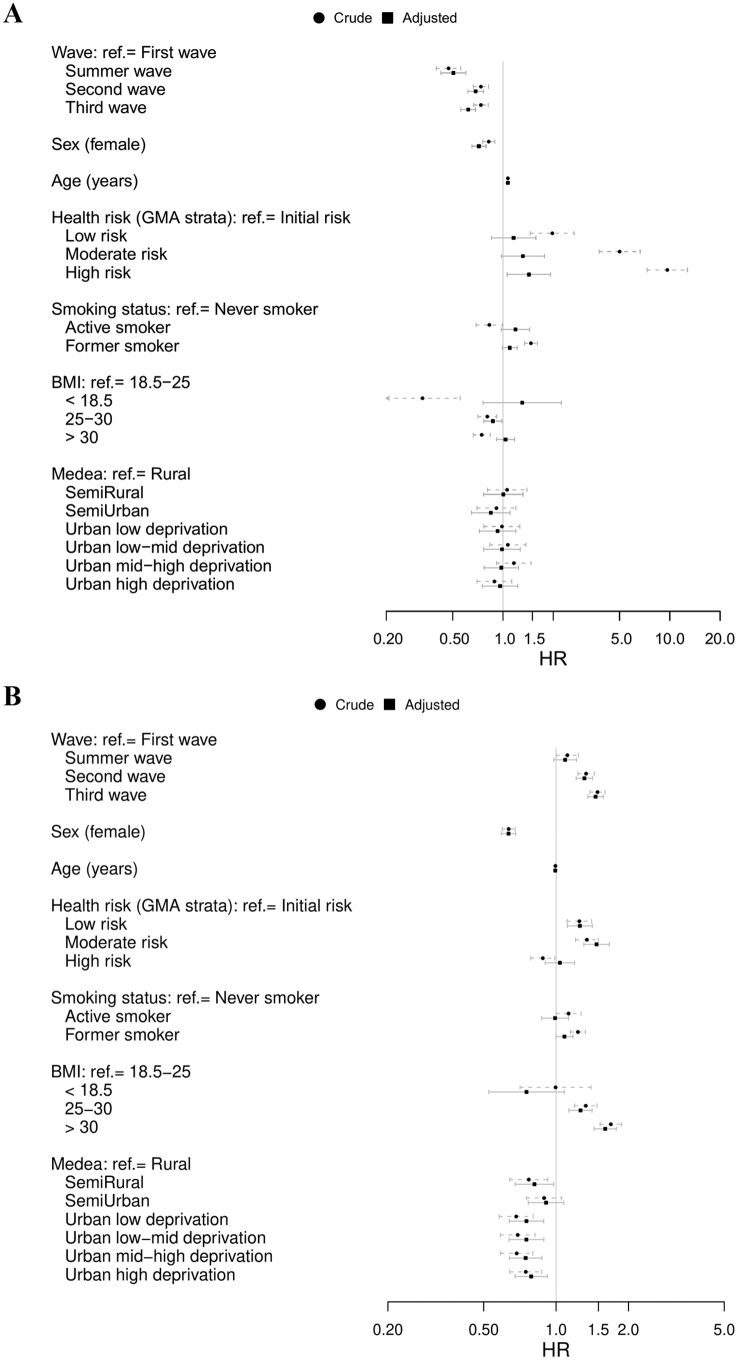
Using the first wave as a comparator, the Cox regression analysis for the overall study sample revealed a 50% reduction of 30-day mortality risk during the summer wave (adjusted HR 0.50; 95% CI 0.43–0.60) and 30% reduction during the second and third waves: adjusted HR 0.69 (0.62–0.77) and 0.62 (0.56–0.69), respectively (Fig. 3). Age, sex, diabetes, chronic kidney disease, and neoplasms were additional risk factors for dying within the 30 days following admission due to COVID-19 after adjusting for all factors.
Fig. 3.
Cox proportional-hazard model for hospital outcomes during the investigated period (fully adjusted model with 10 multiple imputations). A: 30-day mortality. B: transfer to intensive care unit. Horizontal bars show the 95% confidence interval of the hazard ratio, adjusted (continuous line) and unadjusted (dashed grey line). The corresponding models with age included as restricted cubic spline for linearity is provided in Fig. S3 (Supplementary file 1).
The risk of transfer to ICU was significantly higher during the second and third waves (Fig. 3). Other factors associated with ICU transfer included high BMI and GMA health risk higher than baseline. Higher deprivation levels in urban areas were associated with a lower risk of ICU transfer.
Discussion
Our longitudinal analysis showed that some presenting features previously described for hospitalized COVID-19 patients [[7], [8], [9],35] remain consistent throughout various waves of the outbreak, particularly age, sex, and the prevalence of comorbidities like hypertension and diabetes. A remarkable exception to this trend was the milder wave reported during the summer period in our area, which was characterized by younger patients with lower comorbidity burden. Although we could not collect data regarding patients' occupation, these differences might be explained by the local outbreaks reported among seasonal workers, including migrant individuals in vulnerable conditions and more physical contacts [36]. This hypothesis is consistent with the high proportion of individuals from semi-rural areas.
The shift in the presenting characteristics was also translated to changes in mortality, which was particularly lower during the summer wave, presumably due to the younger age of the population during that wave. A meta-analysis investigating mortality of hospitalized COVID-19 patients revealed very high heterogeneity among 33 studies reporting hospital outcomes during the first wave of the pandemic [37]. The pooled mortality for the global population of hospitalized patients (17%, 95% CI 13% - 23%) was very similar to the 30-day mortality observed in our analysis during the first wave (17%). This value decreased — and remained stable — during the second and third waves, suggesting that 13% mortality among hospitalized patients better represents the actual mortality expected for an outbreak wave. Neverthless, the mortality decline to 8% during the summer period (below the 95% CI reported in the pooled analysis) suggests that changes in the transmission pattern (e.g., more people outdoors) [38] and the characteristics of COVID-19 patients may strongly affect mortality. Likewise, vaccination campaigns are expected to shift the profile of hospitalized COVID-19 patients and mark a turning point in mortality in this setting.
Besides the presenting features, hospital outcomes must be placed in the context of hospital capacity and healthcare pathways implemented throughout the pandemic. Like many other countries worldwide [39,40], we experienced a severe overburden of the healthcare system, including hospital resources, during the first wave of the outbreak. The limited hospital resources during the first wave may explain the lower transfer rate to ICU and special units during these period — despite higher mortality — compared with the second and third waves, as shown in our competing risk analysis. This effect, along with the less accurate prescription and therapeutic behaviours in the early stages of the pandemic, is likely to blur the relative weight of factors with potential influence on mortality during the first wave.
Finally, we observed a remarkable increase in the proportion of socioeconomically deprived patients being admitted to hospital after the first wave. To date, various authors have consistently reported the socioeconomic gradient in COVID-19 incidence in the study area [41,42]. Limited access to personal outdoor space, overcrowding, and employments with limited opportunities to work from home have been listed among features that may increase the exposure of economically disadvantaged people to COVID-19 [43,44]. Although our analysis focused on hospitalized patients, the significant shift towards lower socioeconomic levels suggests a broadening of social inequalities in the post-lockdown scenario.
Our analysis was limited by the use of administrative databases, which depend on the accuracy and exhaustivity of data collected during routine practice. An example of this limitation is the BMI, with high rate of missing value because it is unfrequently measured in healthy individuals. This effect might be aggravated by the overwhelming experienced by healthcare professionals, particularly during the first wave. A remarkable example of lack of reporting was the use of oxygen therapy at admission, scarcely reported in medical records in our area, particularly during the first wave. Likewise, retrospective analysis based on electronic records may lose sight on transfers to other hospitals and be affected by a delay in the reporting of diagnostics and procedures. Despite these limitations, our analysis is based on a large sample of patients admitted to hospitals with broad coverage of the population in Catalonia that was likely to capture the heterogeneity of hospitalized individuals from the general population.
In summary, our study has a number of novel findings. We uniquely present data for 4 waves of the pandemic in a large cohort of hospitalized patients. Our analysis show that despite consistent presenting characteristics of COVID-19 patients, mortality rates among hospitalized patients have decreased after the first wave of the outbreak. Notably, socioeconomic disparities tended to increase as the pandemic progresses. Changes in the SARS-CoV-2 spreading patterns, public health measures, and in-hospital management of COVID-19 patients are likely to contribute to differences in mortality among hospitalized patients. Public health interventions aimed at reducing COVID-19 mortality must therefore tackle the elder and the poor and be continuously updated to reflect the evolving patterns of the pandemic.
Funding
This work was partially funded by the grant COVID19 6_17 (to Francesc Vidal), Direcció General de Recerca i Innovació en Salut (Departament de Salut) and BIOCAT, Generalitat de Catalunya.
Declaration of competing interest
KK is a member of the UK Scientific Advisory Group for Emergencies. The rest of the authors have no conflicts of interest to declare.
Acknowledgements
The authors would like to thank Dr. Gerard Carot-Sans (PhD) for providing medical writing assistance during the preparation of the manuscript, Dr. Sònia Abilleira (PhD) for her insightful advice on study design, Josep Basora for his general support in study conduct; Antoni Manuel Fuentes, Eduardo Hermosilla, and Manuel Medina for their technical support in data access. KK is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre (BRC).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gloepi.2022.100071.
Appendix A. Supplementary data
Supplementary material
References
- 1.European Centre for Disease Prevention and Control (ECDC) Covid-19 Situation Dashboard. https://qap.ecdc.europa.eu/public/extensions/COVID-19/COVID-19.html [Internet] [cited 2020 Aug 12]. Available from:
- 2.Flaxman S. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature [Internet] 2020;584:257–261. doi: 10.1038/s41586-020-2405-7. https://pubmed.ncbi.nlm.nih.gov/32512579/ [cited 2020 Aug 12]; Available from: [DOI] [PubMed] [Google Scholar]
- 3.Nafilyan V. Ethnic differences in COVID-19 mortality during the first two waves of the coronavirus pandemic: a nationwide cohort study of 29 million adults in England. medRxiv [Internet] 2021 Feb 5 doi: 10.1101/2021.02.03.21251004. [cited 2021 Apr 16];2021.02.03.21251004. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ [Internet]. [cited 2021 Apr 16]. Available from:
- 5.Bachmann M.F. SARS-CoV-2 structural features may explain limited neutralizing-antibody responses. npj Vaccines. 2021 Dec 1;6(1):1–4. doi: 10.1038/s41541-020-00264-6. https://pubmed.ncbi.nlm.nih.gov/33398006/ [Internet]. [cited 2021 Feb 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contreras S. Risking further COVID-19 waves despite vaccination. Lancet Infect Dis [Internet] 2021;21:745–746. doi: 10.1016/S1473-3099(21)00167-5. [Mar [cited 2021 Apr 8]; Available from: /pmc/articles/PMC7972303/] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argenzian M.G. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ [Internet] 2020;369:m1999. doi: 10.1136/bmj.m1996. [cited 2020 Sep 29];369. Available from: /pmc/articles/PMC7256651/?report=abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson S. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA - J Am Med Assoc. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burn E. The natural history of symptomatic COVID-19 during the first wave in Catalonia. Nat Commun. 2021;12:777. doi: 10.1038/s41467-021-21100-y. [cited 2020 Aug 13];109:2020.07.13.20152454. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turcotte J.J. Risk factors for severe illness in hospitalized Covid-19 patients at a regional hospital. PLoS One [Internet] 2020 Aug 1;15(8 August) doi: 10.1371/journal.pone.0237558. [cited 2020 Sep 29]. Available from: /pmc/articles/PMC7423129/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrilli C.M. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in new York City: prospective cohort study. BMJ [Internet] 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasselli G. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA. Intern Med. 2020:1–11. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Valle D.M. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med [Internet] 2020 Aug 24;3:1–8. doi: 10.1038/s41591-020-1051-9. [cited 2020 Sep 30]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fumagalli C. Clinical risk score to predict in-hospital mortality in COVID-19 patients: a retrospective cohort study. BMJ Open [Internet] 2020;10(9):e040729. doi: 10.1136/bmjopen-2020-040729. Sep 25 [cited 2020 Sep 30];10(9):e040729. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young B.E. Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: an observational cohort study. Lancet (London, England) [Internet] 2020 Aug 18;396(10251):603. doi: 10.1016/S0140-6736(20)31757-8. http://www.ncbi.nlm.nih.gov/pubmed/32822564 [cited 2020 Sep 2]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskier D. Mutation density changes in SARS-CoV-2 are related to the pandemic stage but to a lesser extent in the dominant strain with mutations in spike and RdRp. PeerJ [Internet] 2020;8:e9703. doi: 10.7717/peerj.9703. [Aug 19 [cited 2020 Sep 17];8:e9703. Available from: /pmc/articles/PMC7443079/?report=abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellinghaus D. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N Engl J Med [Internet] 2020 Oct;383(16):1522–1534. doi: 10.1056/NEJMoa2020283. 15 [cited 2020 Oct 21]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islam N. Physical distancing interventions and incidence of coronavirus disease 2019: Natural experiment in 149 countries. BMJ [Internet] 2020 Jul 15;370:2743. doi: 10.1136/bmj.m2743. [cited 2021 Apr 16]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salyer S.J. The first and second waves of the COVID-19 pandemic in Africa: a cross-sectional study. Lancet (London, England) [Internet] 2021 Mar 24;397:10281. doi: 10.1016/S0140-6736(21)00632-2. http://www.ncbi.nlm.nih.gov/pubmed/33773118 [cited 2021 Apr 8]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva C.J. Complex network model for COVID-19: Human behavior, pseudo-periodic solutions and multiple epidemic waves. J Math Anal Appl. 2021 doi: 10.1016/j.jmaa.2021.125171. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soriano V. Main differences between the first and second waves of COVID-19 in Madrid, Spain. Int J Infect Dis [Internet] 2021;105:374–376. doi: 10.1016/j.ijid.2021.02.115. [Apr [cited 2021 Apr 8];105:374–6. Available from: /pmc/articles/PMC7934652/] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karagiannidis C. Major differences in ICU admissions during the first and second COVID-19 wave in Germany. Lancet Respir Med [Internet] 2021 Mar;9(5):e47–e48. doi: 10.1016/S2213-2600(21)00101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James N. Covid-19 second wave mortality in europe and the United States [Internet] Chaos. 2021;31(3):031105. doi: 10.1063/5.0041569. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. Spain [Internet] 2020. https://covid19.who.int/region/euro/country/es [cited 2020 Oct 9]. Available from:
- 25.National Center of Epidemiology COVID-19 in Spain [Internet] https://cnecovid.isciii.es/covid19/#ccaa [cited 2021 Nov 18]. Available from:
- 26.National Cancer Institute Joinpoint Trend Analysis Software, Version 4.8.0.1 [Internet]. Statistical Methodology and Applications Branch, Surveillance Research Program. https://surveillance.cancer.gov/joinpoint/ [cited 2020 Jul 15]. Available from:
- 27.Del Mar García-Gil M. Construction and validation of a scoring system for the selection of high-quality data in a Spanish population primary care database (SIDIAP) Inform Prim Care [Internet] 2012;19(3):135–145. doi: 10.14236/jhi.v19i3.806. https://hijournal.bcs.org/index.php/jhi/article/view/806 [cited 2020 Oct 1]. Available from: [DOI] [PubMed] [Google Scholar]
- 28.Domínguez-Berjón M.F. Construcción de un índice de privación a partir de datos censales en grandes ciudades españolas (Proyecto MEDEA) Gac Sanit [Internet] 2008;22(3):179–187. doi: 10.1157/13123961. https://pubmed.ncbi.nlm.nih.gov/18579042/ May 1 [cited 2021 May 7. Available from. [DOI] [PubMed] [Google Scholar]
- 29.Gotsens M. Socio-economic inequalities in mortality due to injuries in small areas of ten cities in Spain (MEDEA project) Accid Anal Prev. 2011 Sep 1;43(5):1802–1810. doi: 10.1016/j.aap.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Dueñas-Espín I. Proposals for enhanced health risk assessment and stratification in an integrated care scenario. BMJ Open. 2016;6(4) doi: 10.1136/bmjopen-2015-010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monterde D. Adjusted morbidity groups: a new multiple morbidity measurement of use in primary care. Aten Primaria. 2016;48(10):674–682. doi: 10.1016/j.aprim.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) Defining Adult Overweight and Obesity [Internet] https://www.cdc.gov/obesity/adult/defining.html [cited 2020 Aug 10]. Available from:
- 33.Grambsch P.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994 Aug;81(3):515. [Google Scholar]
- 34.Harrell F.E. vol. 608. Springer; 2001. Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. [Google Scholar]
- 35.Suleyman G. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw open [Internet] 2020 doi: 10.1001/jamanetworkopen.2020.12270. [Jun 1 [cited 2020 Sep 29];3(6):e2012270. Available from: /pmc/articles/PMC7298606/?report=abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guzmán Herrador B.R. COVID-19 outbreaks in a transmission control scenario: Challenges posed by social and leisure activities, and for workers in vulnerable conditions, Spain, early summer 2020. Eurosurveillance [Internet] 2020 Sep 1;25(35) doi: 10.2807/1560-7917.ES.2020.25.35.2001545. [cited 2021 Apr 9]. Available from: /pmc/articles/PMC7472688/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macedo A. COVID-19 fatality rates in hospitalized patients: systematic review and meta-analysis. Ann Epidemiol [Internet] 2021;57:14–21. doi: 10.1016/j.annepidem.2021.02.012. [May 1 [cited 2021 Apr 9];57:14–21. Available from: /pmc/articles/PMC7920817/] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulfone T.C. Outdoor transmission of SARS-CoV-2 and other respiratory viruses: a systematic review. J Infect Dis [Internet] 2021;223(4):550–561. doi: 10.1093/infdis/jiaa742. Feb 24 [cited 2021 Apr 18. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hick J.L. National Academy of Medicine; 2020. Duty to Plan: Health care, crisis standards of care, and novel coronavirus SARS-CoV-2 [Internet]. NAM perspectives.https://nam.edu/duty-to-plan-health-care-crisis-standards-of-care-and-novel-coronavirus-sars-cov-2/ [cited 2020 Oct 1]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mannucci E. Saturation of critical care capacity and mortality in patients with the novel coronavirus (COVID-19) in Italy. Trends Anaesth Crit Care [Internet] 2020;33:33–34. doi: 10.1016/j.tacc.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baena-Díez J.M. Impact of COVID-19 outbreak by income: hitting hardest the most deprived. J Public Health (Bangkok) [Internet] 2020;42(4):698–703. doi: 10.1093/pubmed/fdaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amengual-Moreno M. Social determinants of the incidence of Covid-19 in Barcelona: a preliminary ecological study using public data. Rev Esp Salud Publica. 2020;94(94) [PubMed] [Google Scholar]
- 43.Patel J.A. Poverty, inequality and COVID-19: the forgotten vulnerable. Public Health [Internet] 2020;183:110–111. doi: 10.1016/j.puhe.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nafilyan V. Ethnicity, household composition and COVID-19 mortality: A national linked data study [Internet] medRxiv. 2020 doi: 10.1177/0141076821999973. SAGE PublicationsSage UK: London, England. [cited 2021 Apr 18]. p. 014107682199997. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material