Abstract
Hypertension (HT) is an important risk factor for heart failure (HF). The prevalence of HT among the HF population is higher in Asia than in other regions around the world. In Asia, HT is the most common cause of HF after ischemic heart disease. Hypertensive HF (HHF) results from structural and functional adaptations of the heart, which lead to left ventricular (LV) hypertrophy (LVH). Hypertensive LVH can cause ventricular diastolic dysfunction and becomes a risk factor for myocardial infarction, which is a well-known cause of LV systolic dysfunction. Asymptomatic systolic and diastolic LV dysfunction easily progress to clinically overt HF with other precipitating factors. Although the precise pathophysiology of HHF is still unclear, we have known that HHF can be reversed by effective control of blood pressure (BP). Thus, HT control is essential not only for primary prevention but also for the secondary prevention of HF. Here, we reviewed the epidemiology, pathophysiology, outcome, and implication of BP management in HHF patients, especially in the Asian population.
Keywords: Asia, Blood pressure control, Heart failure, Hypertension, Hypertensive heart failure
Background
Heart failure (HF) is a clinical syndrome that has a huge impact on the hospitalization and mortality of the patients [1, 2]. Indeed, 1-year all-cause death rates among HF patients in South Asia, Northeast Asia, and Southeast Asia reach 7.5, 7.4, and 13%, respectively, according to the Asian sudden cardiac death in HF registry (ASIAN-HF) [3]. Also, several HF reports suggest higher mortality in Asian people than in Westerners [4].
Hypertension (HT) is a significant modifiable risk factor for cardiovascular disease (CVD), particularly HF [5, 6, 7]. The causative association of HT with HF has been continuously observed since the first report from the Framingham heart study [6]. This study presents the lifetime risk of HF based on subjects' age and blood pressure (BP). HT doubled the new-onset HF in men and tripled in women after adjusting other risk factors [6]. In the same manner, HT was proven to contribute to existing HF development by 39% in men and 59% in women [6].
Chronic exposure to high BP leads to various alterations in the structure of the myocardium, the heart's conduction system, and coronary vascularization [8]. These changes refer to hypertensive heart disease conditions [8]. Hypertensive heart disease is characterized by a prolonged increase in left ventricular (LV) filling pressure and diastolic dysfunction. The increase in left atrium size in this setting results in impaired LV compliance followed by LV systolic dysfunction in the hypertrophic ventricle [9]. Further LV myocardial damage by myocardial ischemia and arrhythmias accelerates the development of hypertensive HF (HHF) [8].
The American College of Cardiology Foundation and American Heart Association regard HT as a preceding factor for HF and denominate HT without structural heart disease state as stage A HHF [5]. As for stage B HHF, the cardinal sign of systemic arterial HT is LV hypertrophy (LVH) [5, 9]. In addition to LVH, subclinical coronary artery disease (CAD) and silent myocardial infarction (MI) are common in hypertensive patients [5, 8, 10]. The clinical symptoms of stage C HHF patients do not differ from those of patients with HF of other causes. HT is present in most patients who develop HF, especially in HF patients with preserved LV ejection fraction (HFpEF) [5, 8]. Stage D HHF refers to patients with chronic HF that continuously progress and develop severe symptoms despite adequate management, including guideline-directed medical therapy [5].
Epidemiology of HHF in Asia
Most of the HF epidemiologic data up to date came from Europe and North America [11]. There are few data on the epidemiology of HF or the relationship between HF and HT in Asia. In Korea, the prevalence of HF among general population was reported as 1.53% [12]. However, the prevalence continuously increased approximately 2 folds from 2002 to 2013. The prevalence of HF in Taiwan and China was reported as 6% [11] and 1.3% [13], respectively. The prevalence of HF in Japan was relatively low (<1%) than in other Northeast Asia regions [13, 14]. The prevalence of HF in Southeast Asia showed variable prevalences such as 5% in Indonesia, 0.4% in Thailand, and 1–2%, in the Philippines [11].
The characteristics of HF in various registries were summarized in Table 1. According to the Asian Sudden Cardiac Death in HF registry (ASIAN-HF) and the Acute Decompensated HF Registry International-Asia Pacific (ADHERE-AP), Asian-HF patients were almost a decade younger compared to patients in Europe and North America [3, 15, 16]. In the ASIAN-HF registry, Northeast Asian-HF patients are generally older, and the mean body mass index was lower than those of South Asian and Southeast Asian. The oldest HF patients were from Hong Kong, with an average of 73 years old, while the youngest was from the Philippines of 56 years [3]. Similar to the ASIAN-HF registry, ADHERE-AP is an observational study from 8 Asia-Pacific countries between 2006 and 2008. Among HF patients, 40% had HT. In conjunction with ASIAN-HF, data from Southeast Asia registries, such as the Dysfunction Established and Registered adult symptomatic HF (The DEAR Heart) from Philippines, and Indonesia, and Thailand as part of the ADHERE-AP registry showed that Southeast Asian-HF patients were younger than other regions [17, 18, 19]. In contrast, the Korean Acute HF Registry (KorAHF), the Acute Decompensated HF Syndrome registry (ATTEND) from Japan, and Asian-HF registry from Northeast Asia showed the mean onset of HF relative older than other Asian regions [15, 20, 21]. ATTEND registry compared hypertensive and nonhypertensive in hospitalized HF patients after matched age and sex data. The HF patients with HT were older and had more comorbidities such as diabetes mellitus (DM) [21].
Table 1.
Brief summary of the characteristics of HF registries
| Registry | ASIAN-HF [15] | KorAHF [20] | ATTEND [21] | TSOC-HFrEF [26] | ADHERE-AP [16] | THFR [24] | DEAR heart [17] | INTER-CHF [25] | ESC-HF-LT [72] | ADHERE [23] | ||||||||
| Region | Asia | South Asia | Northeast Asia | Southeast Asia | Northeast Asia | Northeast Asia | Northeast Asia | Southeast Asia | Southeast Asia | Asia-Pacific | South Asia | Southeast Asia | Asia, Africa, Middle East, Latin America | Non-Asia | Non-Asia | |||
| Country | Korea | Japan | Taiwan | Indonesia [19] | Thailand [18] | Asia-Pacific | India | Philippines | Overall | India | Southeast Asia | China | European | USA | ||||
| Years | 2012 | 2011 | 2007 | 2013 | 2006 | 2006 | 2006 | 2013 | 2002 | 2012 | 2011 | 2002 | ||||||
| −2015 | −2014 | −2011 | −2014 | −2008 | −2007 | −2008 | −2004 | −2014 | −2013 | −2004 | ||||||||
| Sample size | 5,276 | 1,436 | 1,658 | 2,182 | 5,625 | 4,842 | 1,509 | 1,687 | 2,401 | 10,171 | 1,205 | 1,078 | 5,823 | 858 | 811 | 991 | 4,449 | 159,168 |
|
| ||||||||||||||||||
| Demographic | ||||||||||||||||||
| Age, years (SD) | 59.6 | 57.8 | 62.1 | 58.9 | 68.5 | 73 | 64 | 60 | 64 | 66 | 61.2 | 60 | 59 | 56 | 57 | 66 | 69.35 | 72.4 |
| (13.1) | (12.5) | (14.5) | (11.9) | (14.5) | (13.8) | (15.8) | (14) | (13.7) | (15) | (15) | (14) | (16) | (12.98) | (14.1) | ||||
| Male, % | 78.2 | 75.70 | 74.90 | 82.30 | 53.20 | 58.00 | 72.4 | 64.50 | 49.60 | 57.00 | 69.21 | 57.00 | 61.00 | 62.00 | 59.00 | 57.00 | 62.60 | 48.40 |
| BMI, kg/m2 | 24.9 | 25.0 | 23.9 | 25.7 | 23.3 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 26 | 23 | 26 | 24 | 28.67 | N/A |
| (5.1) | (4.8) | (4.5) | (5.6) | (3.9) | (6.1) | (6.2) | (6) | (6.3) | (5.39) | |||||||||
|
| ||||||||||||||||||
| Comorbidity | ||||||||||||||||||
| HT, % | 51.9 | 37.9 | 48.1 | 64.2 | 62.2 | 69.4 | 34.5 | 54.8 | 64.8 | 64 | 57.76 | 64 | 64 | N/A | N/A | N/A | 65.6 | 73.9 |
| DM, % | 40.4 | 37.1 | 31.8 | 49.3 | 40 | 33.8 | 44 | 31.2 | 47.3 | 45 | 54.94 | 41 | 29 | 26 | 41 | 19 | 39 | 44 |
| AF, % | 17.9 | 4.2 | 30.2 | 17.40 | 28.50 | 39.6 | 26 | 14.6 | 24 | 24 | 14.69 | N/A | N/A | N/A | N/A | N/A | 44 | 30.9 |
|
| ||||||||||||||||||
| Etiology | ||||||||||||||||||
| Ischemic, % | 47 | 37 | 32 | 65 | 37.6 | 31.1 | 44 | 49.9 | 44.70 | N/A | 72 | 52 | 39 | 46 | 56 | 45 | 53.8 | N/A |
| HT, % | N/A | N/A | N/A | N/A | 4.0 | 17.7 | 4.8 | 54.8 | 12.2 | N/A | 0.91 | 6 | 17 | 14 | 15 | 14 | 8.2 | N/A |
|
| ||||||||||||||||||
| Outcome | ||||||||||||||||||
| In-hospital mortality, % | N/A | N/A | N/A | N/A | 4.8 | 6.4 | 2.4 | 6.7 | 5.5 | 4.8 | 8 | 10 | N/A | N/A | N/A | N/A | 4.90 | 3.7 −3.9 |
| LOS, days | N/A | N/A | N/A | N/A | 9.0 | 30.0 | 8.0 | 7.1 | 7.5 | 6 | 6.0 | 10.0 | N/A | N/A | N/A | N/A | N/A | 4.3 |
HF, heart failure; ADHERE, acute decompensated heart failure national registry; ADHERE-AP, the acute decompensated heart failure registry internationale Asia pacific; ATTEND, the acute decompensated heart failure syndromes registry; ASIAN-HF, Asian sudden cardiac death in heart failure; AF, atrial fibrillation; BMI, body mass index; DM, diabetes mellitus; ESC-HF-LT, European Society of cardiology heart failure long-term registry; HT, hypertension; INTER-CHF, international congestive heart failure; KorAHF, the Korean acute heart failure registry; The DEAR, Heart, dysfunction established and registered adult symptomatic heart failure patients; THFR, the Trivandrum heart failure registry; TSOC-HFrEF, the Taiwan society of cardiology-heart failure with reduced ejection fraction; N/A, information not available; LOS, length of stay.
Regarding sex, women had lower prevalence of HF than men in the general Asian registries (Table 1). The prevalence of HFpEF was similar between both sex, whereas HF with reduced LV ejection fraction (HFrEF) were more common in men in all 3 Asian regions, especially in the Southeast Asia region where men showed two-thirds higher prevalence [3, 15, 21]. Interestingly according to the KorAHF, the prevalence of HF is higher in women, by 1.72 than 1.34% in men [12]. This result is similar to the finding from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with HF (OPTIMIZE HF) and the Acute Decompensated HF National Registry (ADHERE) from North America [22, 23].
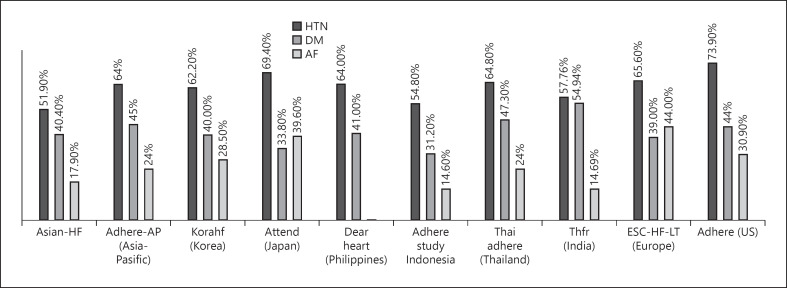
The proportion of HT among the comorbidities of HF varies among Asian countries (Table 1). According to HF cohort registries in Asia, the HT proportion as a comorbidity of HF was between 55 and 69.4%. The highest was 69.4% in Japan, followed by 64.8% in Thailand, 64% in the Philippines, 62.2% in Korea, 57.8% in India, and 54.8% in Indonesia [17, 18, 19, 20, 21, 24]. Similarly, HT is also more prevalent among Western HF population, as data from the European Society of Cardiology HF Long-Term Registry (ESC-HF-LT) reported 65.6% and Acute Decompensated HF National Registry (ADHERE) reported 73.9% of the comorbid proportion. The comorbidities among HF patients are described in Figure 1. HT is the most common chronic noncommunicable disease among HF patients in Asia, Europe, and North America (35–69%), followed by DM and atrial fibrillation (AF).
Fig. 1.
Prevalence of comorbidities among HF registries. ADHERE, Acute Decompensated HEart Failure National Registry; ADHERE-AP, the Acute Decompensated HEart Failure Registry Internationale Asia Pacific; ATTEND, the Acute Decompensated Heart Failure Syndromes registry; ASIAN-HF, Asian Sudden Cardiac Death in Heart Failure; AF, atrial fibrillation; BMI, body mass index; DM, diabetes mellitus; ESC-HF-LT, European Society of Cardiology Heart Failure Long-Term Registry; HT, hypertension; INTER-CHF, International Congestive Heart Failure; KorAHF, the Korean Acute Heart Failure Registry; The DEAR Heart, Dysfunction Established And Registered adult symptomatic Heart failure patients; THFR, the Trivandrum Heart Failure Registry; TSOC-HFrEF, the Taiwan Society of Cardiology-Heart Failure with reduced Ejection Fraction.
HT is reported as the most important cause of HF in Hong Kong and Indonesia, accounting for 70 and 55% of all HF patients, respectively [11, 19]. The prevalence of HT as HF etiology in other regions of Asia such as Japan, China, Thailand, Philippines, Taiwan, and Korea was reported as 17.7, 14, 12.2, 6, 4.8, and 4%, respectively [16, 17, 18, 20, 21, 25, 26]. It is not always easy to define coincident HT as a cause of HF. Many registries do not suggest the definition of HHF in their method section, and only present the prevalence of coincident HT among HF patients or prevalence of HT as HF etiology. In that case, we only reported the proportion of HT as comorbid and cause of HF that presented in some cohort registries.
Pathophysiology and Clinical Characteristic of HHF
The natural course of the HF development among HT patients is well described in the Pressione Arteriose Monitorate E Loro Associazioni (PAMELA) Study [9]. PAMELA study is an epidemiological study designed with the original purpose of determining the normal values of home BP and ambulatory BP monitoring. However, this study showed a valuable natural history of HT progressing to HF. Although the baseline BP level and HT prevalence of the study population were similar to general population, many patients developed LVH after 10 years of follow-up. Especially in hypertensive and prehypertensive patients, there were more than 2-fold increase in LVH [9].
HT increases LV afterload, which is exacerbated by chronicity, severity of BP, and systemic vascular resistance resulting in LV remodeling [27, 28]. This pressure overload causes mechanical stress as a hemodynamic factor. Along with this process, other factors contribute to the progression of HF, namely neurohormonal, cytokine, comorbidities, race, and genetic factors [29]. Paulus and Tschöpe [30] conceived a new concept for the development of HF that occurs in HFpEF patients. It begins with the induction of a systemic inflammatory process due to the patient's comorbid conditions such as HT and obesity. The systemic pro-inflammatory state causes inflammation of the coronary artery endothelium, reducing nitric oxide and protein kinase G activity leading to fibrosis and hypertrophy of cardiomyocytes in the left ventricle. Thereby increased LV stiffness and impaired LV relaxation eventually lead to LV diastolic dysfunction [30]. Diastolic dysfunction may antedate LVH [31] and induce parallel addition of new myofibril, leading to compensatory changes such as relatively increased LV mass and wall thickness [27].
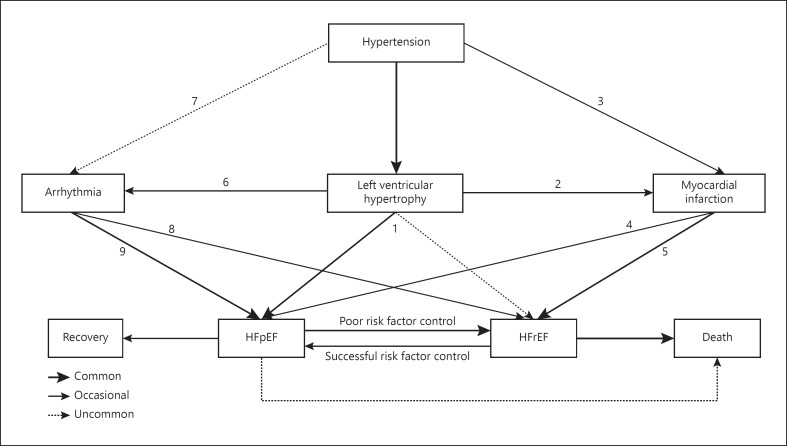
The pathophysiology of HHF is illustrated in Figure 2. Aortic stiffening with HT causes LV diastolic dysfunction in patients with HHF and also rise in central SBP that promotes increase in afterload and myocyte size [32]. In contrast, increased aortic stiffness also reduces diastolic BP (DBP) and compromise coronary perfusion, thus further aggravating subendocardial ischemia and myocardial fibrosis leading to HFpEF condition (Pathway 1) [32]. HFpEF more commonly occurs than HFrEF in HT patients (Pathway 1) [33, 34]. Neurohormones play a role in the LV geometric remodeling in either eccentric or concentric LVH by causing myocyte hypertrophy.
Fig. 2.
Pathophysiology of hypertensive heart failure. HT progresses to LVH. Along with this process, the patient's condition can directly develop to symptomatic HF with preserved ejection fraction (HFpEF) or HF with reduced ejection fraction (HFrEF) (pathway 1). Hypertensive patients with progression to LVH (pathway 2) or without LVH (pathway 3) who suffer MI may develop to HFpEF (pathway 4), and more prevalent develop to HF with reduced ejection fraction (HFrEF) (pathway 5). HT is associated with a variety of cardiac arrhythmias, most AF. Arrhythmias may occur in hypertensive patients, commonly with LVH progression than without LVH (pathway 6 and 7). AF is more common to develop HFpEF (pathway 8) than to HFrEF (pathway 9). HFpEF patients who commonly suffer MI and poor risk factor control develop HF with reduced ejection fraction (HFrEF). HFrEF patients with successful risk factor control and treated with evidence-based medication may improve to HFpEF condition. A thicker arrow shows a more common pathway compared with a thinner arrow. HT, hypertension; LVH, left ventricular hypertrophy; AF, atrial fibrillation; MI, myocardial infarction.
Differences in renin activity levels affect LV remodeling in hypertensive patients. Researchers found that patients with concentric LVH had high renin activity [35] and the opposite for eccentric LVH [36]. Demographic differences also affect the relationship between HT and type of LV remodeling. Women are more likely to exhibit concentric LVH, while men are more likely to exhibit eccentric LVH [37]. Levy et al. [38], along with the Framingham heart study (FHS), found that in both sexes, the increase in SBP was significantly associated with LVH. After adjusting with CAD risk factor, LVH was significantly associated with MI (Pathway 2). In the combined analysis of 4 prospective community-based cohorts, including the FHS, the Prevention of Renal and Vascular Endstage Disease (PREVEND), the Cardiovascular Heart Study (CHS), and the Multi-Ethnic Study of Atherosclerosis (MESA), hypertensive patients who experienced MI (Pathway 2 and pathway 3) may more frequently develop HFpEF (Pathway 4), and HFrEF (Pathway 5) [29, 39, 40, 41, 42]. HT also can be associated with arrhythmia, primarily AF (Pathways 6 and 7) [43]. Increased secretion of angiotensin II induces the proliferation of fibroblast, predisposing factor of AF [44]. AF is more prevalent among patients with HFpEF (Pathway 9) than those with HFrEF (Pathway 8) [43]. If MI occurs in HFpEF patients, they might develop HFrEF [29].
Dunlay et al. [45], in their community-based cohort study in Olmsted County, analyzed longitudinal changes in EF in HFpEF and HFrEF patients. 39% of the HFpEF patients experienced further EF reduction to <50% and thus developed HFrEF, and a similar proportion of HFrEF patients (39%) had improved EF to ≥50% during the follow-up period [45]. In those with reduced EF, EF decreased by 5.8% over 5 years with bigger reduction in older patients and patients with CAD. In contrast, in HFrEF patients, EF by 6.9% over 5 years and more in younger patients, female sex, those treated with evidence-based medication, and those without CAD [45]. Among HFpEF patients, a reduction of 5% in EF was associated with a 7% increase in mortality. Conversely, in HFrEF patients, an increase of 5% in EF was associated with a 12% mortality reduction [45]. This finding is consistent with other studies on HF patients with improved ejection fraction (HFiEF), such as the Valsartan HF Trial (Val-HeFT) [46] and KorAHF study [47]. After 12 months of follow-up, in the Val-HeFT trial, 9.1% of patients from HFrEF group moved to the HFiEF (EF >40%) subgroup and 31.3% of HFrEF patients from the KorAHF study improved and moved to HFiEF group [46, 47]. Patients in HFiEF group were significantly better in mortality and prognosis than those who persistently remained in HFrEF group in these studies [46, 47]. In the ASIAN-HF study, HFpEF patients showed better mortality outcomes than HFrEF patients [3].
Outcome and Therapeutic Implication of HHF
HF is associated with grave prognosis. According to HF registries data in Asia, the length of stay in hospital ranged from 6 to 30 days, and in-hospital mortality ranged 2.4–10% (Table 1). Non-Asian registries showed fewer in-hospital deaths of 3.7–4.9% and shorter length of stay (4.3 days) [23, 48]. The 1-year mortality rate in the Southeast Asia region was similarly reported in the ASIAN-HF study and INTER-CHF study, as around 13–15% [3, 25]. In contrast, data from South Asia and Northeast Asia registries showed lower mortality of 7.5 and 7.4%, respectively [3]. In the KorAHF registry, post-discharge 30-day mortality was 3.3% [20]. 1-, 2-, and 3-year mortality was 18.2%, 27.6%, and 34.7%, respectively [20]. In ATTEND registry from Japan, 1-year all-cause death rate was 18.4%, and cardiac death rate was 11.5% [21].
The independent predictors of morbidity and mortality in HF patients include age, comorbidities, SBP, renal function, serum sodium, hemoglobin, natriuretic peptide concentration, troponin, QRS duration, and evidence-based medication utilization [49]. Data from ADHERE (Acute Decompensated HF National Registry) and OPTIMIZE-HF registries found SBP and renal function at admission were among the best discriminators between hospital survivors and nonsurvivors [22, 23]. The OPTIMIZE-HF registry found that 8 factors, including age, weight, SBP, sodium, serum creatinine, and comorbid disease states, could predict the combined endpoint of death or readmission with a c-index of 0.72 [22].
BP control target becomes the most crucial discussion among HT experts for primary prevention of HF development [5, 50] and secondary prevention of death or repeated hospitalization in established HF patients [2, 51, 52, 53, 54]. The recent European guidelines strongly suggest that lowering office SBP/DBP to <140/90 mm Hg is beneficial for all patients groups but recommend further reduction of SBP/DBP under 130/80 mm Hg in high-risk patients, including patients with HF [51]. The 2017 ACC/American Heart Association HT guideline recommends a BP target of <130/80 mm Hg in adults with known CVD or moderate-to-high CVD risk as primary prevention [55]. The Korean Society of HT guideline, a representative guideline for the Asian population, recommends BP target of <140/90 mm Hg for uncomplicated HT in the general population including the elderly, and more strict BP target of <130/80 mm Hg is recommended for those who previously suffered from cerebrovascular disease, chronic kidney disease with albuminuria, diabetes, and CVD [56].
Management of HT in patient with HHF is challenging. In the KorAHF registry, BP and HF mortality showed inverse J-curve shape relationship. The mortality was the lowest with BP of 132/74 mm Hg and increased with further decrease in BP toward 130/70 mm Hg [57]. This finding is consistent with the recommendations of The Korean Society of HT guidelines, which reports an optimal BP is 130/80 mm Hg in HHF patients [56]. However, the evidence describing optimal BP in patients with HF is limited.
The effect of the BP-lowering in primary prevention of HF is presented in Table 2. According to the Systolic Blood Pressure Intervention Trial (SPRINT), 9,361 random people with SBP over 130 mm Hg without DM were randomly assigned to intensive SBP control group (target SBP <120 mm Hg) or standard control group (target SBP <140 mm Hg). After a mean follow-up of 3.3 years, the mean SBP in the intensive arm and the standard arm were 121.5 and 134.6 mm Hg, respectively. Intensive BP-lowering markedly reduced the incidence of the primary outcome by around 40%, with 38% reduction of acute decompensated HF; 27% reduction of all-cause death [8, 50]. In the Hypertension in the Very Elderly Trial (HYVET), BP-lowering agents reduced HF development by 64% compared to placebo. A meta-analysis of 35 HT randomized control trials by Thomopoulos et al. [59] showed 37% relative risk reduction in HF primary prevention by antihypertensive agents use. SBP, DBP, and pulse pressure reduction all had significant and robust correlation with HF prevention [59]. These findings highlight the importance of BP control in the prevention of HF development. While the BP-lowering strategy from the SPRINT trial showed clear benefit, the Asian population was not included in the study, leaving the necessity for different BP threshold in Asian patients. Therefore, further studies in Asian hypertensive patients should be warranted for establishing optimal BP for the prevention of HHF.
Table 2.
Summary of the BP-lowering effect in HF prevention (primary prevention) [49, 58–60, 67, 73]
| Disease status | Intervention (BP target or drugs) | Findings | Reference number |
|---|---|---|---|
| HT | HYVET: active-treatment group versus placebo active-treatment (indapamide and perindopril) SBP target <150/80 mm Hg | RR reduction of fatal and nonfatal HF by 64% in active-treatment arm | [67] |
|
| |||
| SPRINT: intensive control versus standard control SBP target <120 mm Hg versus <140 mm Hg | RR reduction of HF by 38% in intensive control arm | [49] | |
|
| |||
| HT | SPRINT: intensive control versus standard control SBP target <120 mm Hg versus <140 mm Hg | RR reduction of new AF by 26% in intensive arm | [58] |
|
| |||
| HT | SPRINT: intensive control versus standard control SBP target <120 mm Hg versus <140 mm Hg | No difference in LVM index between intensive versus standard arm (mean ± SE −2.7 ± 0.5 g vs. −2.3 ± 0.7 g; p = 0.368) | [73] |
|
| |||
| LIFE trial: losartan versus atenolol | Greater reduction in LVM index between losartan versus atenolol (−21.7 ± 21.8 vs. −17.7 ± 19.6 g/m2; p = 0.021) | [59] | |
|
| |||
| Meta-analysis: ACEi versus ARB versus β-blocker versus CCB versus diuretic | Greater reduction in LVM index between ARB, ACEi, CCB versus β-blocker | [60] | |
BP, blood pressure; AF, atrial fibrillation; ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; β-blocker, beta-blockers; CCB, calcium channel blockers; CI, confidence interval; HF, heart failure; HYVET, the hypertension in the very elderly trial; LIFE, the losartan intervention for endpoint reduction in hypertension trial; LVM, left ventricular mass; RR, relative risk; SBP, systolic blood pressure; SPRINT, systolic blood pressure intervention trial; HT, hypertension.
Besides HF prevention, BP-lowering was beneficial for controlling precursors of HHF. In the substudy of the SPRINT trial, intensive BP control significantly reduced AF incidence by 26% compared to the standard BP control [60]. Losartan Intervention For Endpoint reduction (LIFE) trial showed that losartan, an angiotensin receptor blocker, reduced LV mass index greater than atenolol, a β-blocker. The LV mass index difference between 2 groups was consistent up to 5-year follow-up, with comparable magnitude among annual examination [61]. BP remained similarly low throughout the whole follow-up period, suggesting that antihypertensive agents had long-lasting LVM reducing effects. Angiotensin-converting enzyme inhibitors (ACEIs) and calcium channel blockers were more effective in reducing LVM than β-blockers [62], as well.
The benefit of HF medications with BP-lowering property in HF patients is summarized in Table 3. The Val-HeFT trial compared the effectiveness of valsartan versus placebo in HF patients. Valsartan showed better result, reducing the relative risk for AF by 37% [63]. Standard HF therapy is effective in patients with HFrEF but does not reduce morbidity or mortality in patients with HFpEF [52, 53, 54, 64, 65, 66]. In a meta-analysis by Pinho-Gomes et al. [67], HF medications with BP-lowering properties significantly decreased cardiovascular mortality and HF hospitalization by 10%. Secondary prevention of HHF is not targeting BP reduction but targeting LV reverse remodeling, relief of subjective discomfort, improving functional status, and preventing repeated hospitalization or death [2, 5, 53, 54, 66].
Table 3.
Summary of the benefit of HF medication with BP-lowering property in HF patients (secondary prevention)
| Disease status | Intervention (BP target or drugs) | Findings | Reference number |
|---|---|---|---|
| HFpEF | PARAGON HF: sacubitril valsartan versus valsartan in HFpEF patients | Sacubitril valsartan did not significantly affect primary event rate ratio | [62] |
|
| |||
| CHARM- preserved trial: candesartan versus placebo in HFpEF patients. candesartan SBP 136.3 (baseline) to 129 (during trial) | Lesser patients experienced HF hospitalization in candesartan group versus placebo group (adjusted hazard ratio: 0.84, p = 0.047) | [51] | |
|
| |||
| I-PRESERVE: irbesartan versus placebo in ≥60 years HFpEF patients | Irbesartan did not significantly affect primary event ratio versus placebo | [63] | |
|
| |||
| HFrEF | Val-HeFT: valsartan versus placebo in HFrEF patients | RR reduction of new AF by 37% in valsartan arm | [61] |
|
| |||
| HFrEF | SOLVD: enalapril versus placebo in HFrEF patients | Fewer patients died or were hospitalized for worsening HF in enalapril versus placebo group (risk reduction 26%) | [52] |
|
| |||
| ELITE II study: losartan (ARB) versus captopril (ACEi) in HFrEF patients | Fewer patients died in losartan versus captopril group (estimated annual mortality rate: 11.7% [losartan] vs. 10.4% [captopril]) | [53] | |
|
| |||
| PARADIGM-HF: angiotensin-neprilysin inhibition (LCZ696) versus enalapril in HFrEF patients SBP baseline 122 ± 15 versus 121 ± 15 | Fewer patients had cardiovascular death or hospitalization in LCZ696 group versus enalapril (RR reduction: 20%) | [64] | |
BP, blood pressure; AF, atrial fibrillation; ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; CHARM-Preserved, Candesartan in Heart Failure assessment of reduction in mortality and morbidity and preserved left ventricular ejection fraction; CI, confidence interval; ELITE II, the losartan heart failure survival study; HF, heart failure; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; I-PRESERVE, irbesartan in heart failure with preserved systolic function trial; PARADIGM HF, prospective comparison of arni[angiotensin receptor–neprilysin inhibitor] with acei, to determine impact on global mortality and morbidity in heart failure; PARAGON HF, The prospective comparison of ARNI, with ARB, global outcomes in HF, with preserved ejection fraction; RR, relative risk; SBP, systolic blood pressure; SOLVD, studies of left ventricular dysfunction; SPRINT, systolic blood pressure intervention trial; Val-HeFT, valsartan heart failure trial.
Not all hypertensive medications share beneficial effects for HF. Angiotensin receptor blockers, ACEIs, β-blockers are effective both in primary prevention [59, 68] and secondary prevention of HF, constituting the important components of guideline-directed management and therapy of HF [2, 5]. Diuretics are beneficial for symptom relief and for primary prevention of HF, but it is not clear whether they have a role in secondary prevention [2, 69]. Alpha-blockers do not have a role in HF prevention, but rather have adverse effect in HF prevention, especially for the secondary prevention [70]. Calcium channel blockers are effective BP-lowering medications, but they may increase fluid retention and should be cautiously used in HHF patients [2, 5, 71, 72].
Summary
Among HF patients in Asia, HT is the most prevalent comorbidity and is the common cause of HF. Asian HHF patients are younger than those in the Western countries, with the majority of male patients. The pathophysiology of HHF is complex, which is influenced by many factors (e.g., mechanical stress as a hemodynamic factor, neurohormonal, and comorbidities). HT control is essential for both primary and secondary prevention of HHF. The optimal BP in HHF remains unclear yet, and further research is needed to determine the target BP for primary and secondary prevention of HHF, particularly in Asian population.
Conflict of Interest Statement
The authors declare no conflicts of interest regarding the review article.
Funding Sources
Funding sources are not applicable for this study.
Author Contributions
H.R. is a major contributor to analyzed published data and writing the manuscript. H.Y.L. has contributed in conceptualization of this review and in writing the manuscript.
References
- 1.Shiba N, Nochioka K, Miura M, Kohno H, Shimokawa H, Investigators C. Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan: first report from the CHART-2 study. Circ J. 2011;75((4)):823–33. doi: 10.1253/circj.cj-11-0135. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;37((27)):2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald MR, Tay WT, Teng TK, Anand I, Ling LH, Yap J, et al. Regional variation of mortality in heart failure with reduced and preserved ejection fraction across Asia: outcomes in the ASIAN-HF registry. J Am Heart Assoc. 2020;9((1)):e012199. doi: 10.1161/JAHA.119.012199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mentz RJ, Roessig L, Greenberg BH, Sato N, Shinagawa K, Yeo D, et al. Heart failure clinical trials in east and southeast Asia: understanding the importance and defining the next steps. JACC Heart Fail. 2016;4((6)):419–27. doi: 10.1016/j.jchf.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62((16)):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106((24)):3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 7.Dorobantu MM, Giuseppe MV, Grassi G, Voicu V. Hypertension and heart failure epidemiology, mechanisms and treatment. Switzerland: Springer Nature; 2019. [Google Scholar]
- 8.Kalogeropoulos AP, Goulbourne C, Butler J. Diagnosis and prevention of hypertensive heart failure. Heart Fail Clin. 2019;15((4)):435–45. doi: 10.1016/j.hfc.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Cuspidi C, Sala C, Casati A, Bombelli M, Grassi G, Mancia G. Clinical and prognostic value of hypertensive cardiac damage in the PAMELA Study. Hypertens Res. 2017;40((4)):329–35. doi: 10.1038/hr.2016.153. [DOI] [PubMed] [Google Scholar]
- 10.Soliman EZ. Silent myocardial infarction and risk of heart failure: current evidence and gaps in knowledge. Trends Cardiovasc Med. 2019;29((4)):239–44. doi: 10.1016/j.tcm.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Reyes EB, Ha JW, Firdaus I, Ghazi AM, Phrommintikul A, Sim D, et al. Heart failure across Asia: same healthcare burden but differences in organization of care. Int J Cardiol. 2016;223:163–7. doi: 10.1016/j.ijcard.2016.07.256. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Lim NK, Cho MC, Park HY. Epidemiology of heart failure in Korea: present and future. Korean Circ J. 2016;46((5)):658–64. doi: 10.4070/kcj.2016.46.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1((1)):4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 14.Konishi M, Ishida J, Springer J, von Haehling S, Akashi YJ, Shimokawa H, et al. Heart failure epidemiology and novel treatments in Japan: facts and numbers. ESC Heart Fail. 2016;3((3)):145–51. doi: 10.1002/ehf2.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam CS, Teng TK, Tay WT, Anand I, Zhang S, Shimizu W, et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J. 2016;37((41)):3141–53. doi: 10.1093/eurheartj/ehw331. [DOI] [PubMed] [Google Scholar]
- 16.Atherton JJ, Hayward CS, Wan Ahmad WA, Kwok B, Jorge J, Hernandez AF, et al. Patient characteristics from a regional multicenter database of acute decompensated heart failure in Asia Pacific (ADHERE international-Asia Pacific) J Card Fail. 2012;18((1)):82–8. doi: 10.1016/j.cardfail.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Jorge J, Encarnacion D, Lacson S-M, Lim-Quizon MC, Borromeo A, Abarquez R, et al. The D.E.A.R. heart Program − a registry of adult symptomatic heart failure patients being admitted to tertiary hospitals in the philippines: insights gained from 1078 patients registered in 6 pilot hospitals from Nov. 1, 2002 to Dec. 1, 2004. J Card Fail. 2007;35((1)):32–43. [Google Scholar]
- 18.Laothavorn P, Hengrussamee K, Kanjanavanit R, Moleerergpoom W, Laorakpongse D, Pachirat O, et al. Thai acute decompensated heart failure registry (Thai ADHERE) CVD Prev Control. 2010;5((3)):89–95. [Google Scholar]
- 19.Siswanto BB, Radi B, Kalim H, Santoso A, Suryawan R, Erwinanto, et al. Heart failure in NCVC Jakarta and 5 hospitals in Indonesia. CVD Prev Control. 2010;5((1)):35–8. [Google Scholar]
- 20.Lee SE, Lee HY, Cho HJ, Choe WS, Kim H, Choi JO, et al. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF) Korean Circ J. 2017;47((3)):341–53. doi: 10.4070/kcj.2016.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato N, Kajimoto K, Asai K, Mizuno M, Minami Y, Nagashima M, et al. Acute decompensated heart failure syndromes (ATTEND) registry. A prospective observational multicenter cohort study: rationale, design, and preliminary data. Am Heart J. 2010;159((6)):949–55.e1. doi: 10.1016/j.ahj.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Age- and gender-related differences in quality of care and outcomes of patients hospitalized with heart failure (from OPTIMIZE-HF) Am J Cardiol. 2009;104((1)):107–15. doi: 10.1016/j.amjcard.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 23.Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW, Committee ASA, et al. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2007;153((6)):1021–8. doi: 10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Harikrishnan S, Sanjay G, Anees T, Viswanathan S, Vijayaraghavan G, Bahuleyan CG, et al. Clinical presentation, management, in-hospital and 90-day outcomes of heart failure patients in Trivandrum, Kerala, India: the Trivandrum Heart Failure Registry. Eur J Heart Fail. 2015;17((8)):794–800. doi: 10.1002/ejhf.283. [DOI] [PubMed] [Google Scholar]
- 25.Dokainish H, Teo K, Zhu J, Roy A, AlHabib KF, ElSayed A, et al. Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Glob Health. 2017;5((7)):e665–72. doi: 10.1016/S2214-109X(17)30196-1. [DOI] [PubMed] [Google Scholar]
- 26.Wang CC, Chang HY, Yin WH, Wu YW, Chu PH, Wu CC, et al. TSOC-HFrEF registry: a registry of hospitalized patients with decompensated systolic heart failure: description of population and management. Acta Cardiol Sin. 2016;32((4)):400–11. doi: 10.6515/ACS20160704A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grossman W, Paulus WJ. Myocardial stress and hypertrophy: a complex interface between biophysics and cardiac remodeling. J Clin Invest. 2013;123((9)):3701–3. doi: 10.1172/JCI69830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenchaiah S, Pfeffer MA. Cardiac remodeling in systemic hypertension. Med Clin North Am. 2004;88((1)):115–30. doi: 10.1016/s0025-7125(03)00168-8. [DOI] [PubMed] [Google Scholar]
- 29.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123((3)):327–34. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 30.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62((4)):263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 31.Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kühl U, et al. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol. 2011;57((8)):977–85. doi: 10.1016/j.jacc.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91((12)):1551–6. doi: 10.1136/hrt.2004.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devereux RB, Roman MJ. Left ventricular hypertrophy in hypertension: stimuli, patterns, and consequences. Hypertens Res. 1999;22((1)):1–9. doi: 10.1291/hypres.22.1. [DOI] [PubMed] [Google Scholar]
- 34.Sorrentino MJ. The evolution from hypertension to heart failure. Heart Fail Clin. 2019;15((4)):447–53. doi: 10.1016/j.hfc.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Muscholl MW, Schunkert H, Muders F, Elsner D, Kuch B, Hense HW, et al. Neurohormonal activity and left ventricular geometry in patients with essential arterial hypertension. Am Heart J. 1998;135((1)):58–66. doi: 10.1016/s0002-8703(98)70343-6. [DOI] [PubMed] [Google Scholar]
- 36.du Cailar G, Pasquié JL, Ribstein J, Mimran A. Left ventricular adaptation to hypertension and plasma renin activity. J Hum Hypertens. 2000;14((3)):181–8. doi: 10.1038/sj.jhh.1000974. [DOI] [PubMed] [Google Scholar]
- 37.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72((3)):310–3. doi: 10.1016/0002-9149(93)90678-6. [DOI] [PubMed] [Google Scholar]
- 38.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med. 1989;110((2)):101–7. doi: 10.7326/0003-4819-110-2-101. [DOI] [PubMed] [Google Scholar]
- 39.Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, et al. Predicting heart failure with preserved and reduced ejection fraction: the international collaboration on heart failure subtypes. Circ Heart Fail. 2016;9((6)) doi: 10.1161/CIRCHEARTFAILURE.115.003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34((19)):1424–31. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 41.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275((20)):1557–62. [PubMed] [Google Scholar]
- 42.Peters MN, Seliger SL, Christenson RH, Hong-Zohlman SN, Daniels LB, Lima JAC, et al. “Malignant” left ventricular hypertrophy identifies subjects at high risk for progression to asymptomatic left ventricular dysfunction, heart failure, and death: MESA (multi-ethnic study of atherosclerosis) J Am Heart Assoc. 2018;7((4)):e006619. doi: 10.1161/JAHA.117.006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12((10)):1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 44.Erkuner O, Dudink E, Nieuwlaat R, Rienstra M, Van Gelder IC, Camm AJ, et al. Effect of systemic hypertension with versus without left ventricular hypertrophy on the progression of atrial fibrillation (from the Euro Heart Survey) Am J Cardiol. 2018;122((4)):578–83. doi: 10.1016/j.amjcard.2018.04.053. [DOI] [PubMed] [Google Scholar]
- 45.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5((6)):720–6. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Florea VG, Rector TS, Anand IS, Cohn JN. Heart failure with improved ejection fraction: clinical characteristics, correlates of recovery, and survival: results from the valsartan heart failure trial. Circ Heart Fail. 2016;9((7)):e003123. doi: 10.1161/CIRCHEARTFAILURE.116.003123. [DOI] [PubMed] [Google Scholar]
- 47.Park CS, Park JJ, Mebazaa A, Oh IY, Park HA, Cho HJ, et al. Characteristics, outcomes, and treatment of heart failure with improved ejection fraction. J Am Heart Assoc. 2019;8((6)):e011077. doi: 10.1161/JAHA.118.011077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. 2016;18((6)):613–25. doi: 10.1002/ejhf.566. [DOI] [PubMed] [Google Scholar]
- 49.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63((12)):1123–33. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 50.Group SR, Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373((22)):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al. [2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH)] The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH)] G Ital Cardiol. 2018;19((11 Suppl 1)):3S–73S. doi: 10.1714/3026.30245. [DOI] [PubMed] [Google Scholar]
- 52.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362((9386)):777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 53.Investigators S, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325((5)):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 54.Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial: the losartan heart failure survival study ELITE II. Lancet. 2000;355((9215)):1582–7. doi: 10.1016/s0140-6736(00)02213-3. [DOI] [PubMed] [Google Scholar]
- 55.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71((6)):1269–324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 56.Lee HY, Shin J, Kim GH, Park S, Ihm SH, Kim HC, et al. Korean Society of Hypertension Guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin Hypertens. 2019;25:20. doi: 10.1186/s40885-019-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee SE, Lee HY, Cho HJ, Choe WS, Kim H, Choi JO, et al. Reverse J-curve relationship between on-treatment blood pressure and mortality in patients with heart failure. JACC Heart Fail. 2017;5((11)):810–9. doi: 10.1016/j.jchf.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 58.Upadhya B, Rocco M, Lewis CE, Oparil S, Lovato LC, Cushman WC, et al. Effect of intensive blood pressure treatment on heart failure events in the systolic blood pressure reduction intervention trial. Circ Heart Fail. 2017;10((4)):e003613. doi: 10.1161/CIRCHEARTFAILURE.116.003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering treatment. 6. Prevention of heart failure and new-onset heart failure–meta-analyses of randomized trials. J Hypertens. 2016;34((3)):373–84. doi: 10.1097/HJH.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 60.Soliman EZ, Rahman AF, Zhang ZM, Rodriguez CJ, Chang TI, Bates JT, et al. Effect of intensive blood pressure lowering on the risk of atrial fibrillation. Hypertension. 2020;75((6)):1491–6. doi: 10.1161/HYPERTENSIONAHA.120.14766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Devereux RB, Dahlöf B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110((11)):1456–62. doi: 10.1161/01.CIR.0000141573.44737.5A. [DOI] [PubMed] [Google Scholar]
- 62.Fagard RH, Celis H, Thijs L, Wouters S. Regression of left ventricular mass by antihypertensive treatment: a meta-analysis of randomized comparative studies. Hypertension. 2009;54((5)):1084–91. doi: 10.1161/HYPERTENSIONAHA.109.136655. [DOI] [PubMed] [Google Scholar]
- 63.Maggioni AP, Latini R, Carson PE, Singh SN, Barlera S, Glazer R, et al. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT) Am Heart J. 2005;149((3)):548–57. doi: 10.1016/j.ahj.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 64.Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381((17)):1609–20. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 65.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359((23)):2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 66.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371((11)):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 67.Pinho-Gomes AC, Azevedo L, Bidel Z, Nazarzadeh M, Canoy D, Copland E, et al. Effects of blood pressure-lowering drugs in heart failure: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019;37((9)):1757–67. doi: 10.1097/HJH.0000000000002094. [DOI] [PubMed] [Google Scholar]
- 68.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358((18)):1887–98. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 69.Faris RF, Flather M, Purcell H, Poole-Wilson PA, Coats AJ. WITHDRAWN: diuretics for heart failure. Cochrane Database Syst Rev. 2012;4:CD003838. doi: 10.1002/14651858.CD003838.pub3. [DOI] [PubMed] [Google Scholar]
- 70.Johnson K, Oparil S, Davis BR, Tereshchenko LG. Prevention of heart failure in hypertension-disentangling the role of evolving left ventricular hypertrophy and blood pressure lowering: the ALLHAT study. J Am Heart Assoc. 2019;8((8)):e011961. doi: 10.1161/JAHA.119.011961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Packer M, O'Connor CM, Ghali JK, Pressler ML, Carson PE, Belkin RN, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group. N Engl J Med. 1996;335((15)):1107–14. doi: 10.1056/NEJM199610103351504. [DOI] [PubMed] [Google Scholar]
- 72.Cohn JN, Ziesche S, Smith R, Anand I, Dunkman WB, Loeb H, et al. Effect of the calcium antagonist felodipine as supplementary vasodilator therapy in patients with chronic heart failure treated with enalapril: V-HeFT III. Vasodilator-Heart Failure Trial (V-HeFT) Study Group. Circulation. 1997;96((3)):856–63. doi: 10.1161/01.cir.96.3.856. [DOI] [PubMed] [Google Scholar]
- 73.Upadhya B, Rocco MV, Pajewski NM, Morgan T, Blackshear J, Hundley WG, et al. Effect of Intensive Blood Pressure Reduction on Left Ventricular Mass, Structure, Function, and Fibrosis in the SPRINT-HEART. Hypertension. 2019;74:276–284. doi: 10.1161/HYPERTENSIONAHA.119.13073. [DOI] [PMC free article] [PubMed] [Google Scholar]