Table 2.
Substrate scope of BCPs via intramolecular coupling.
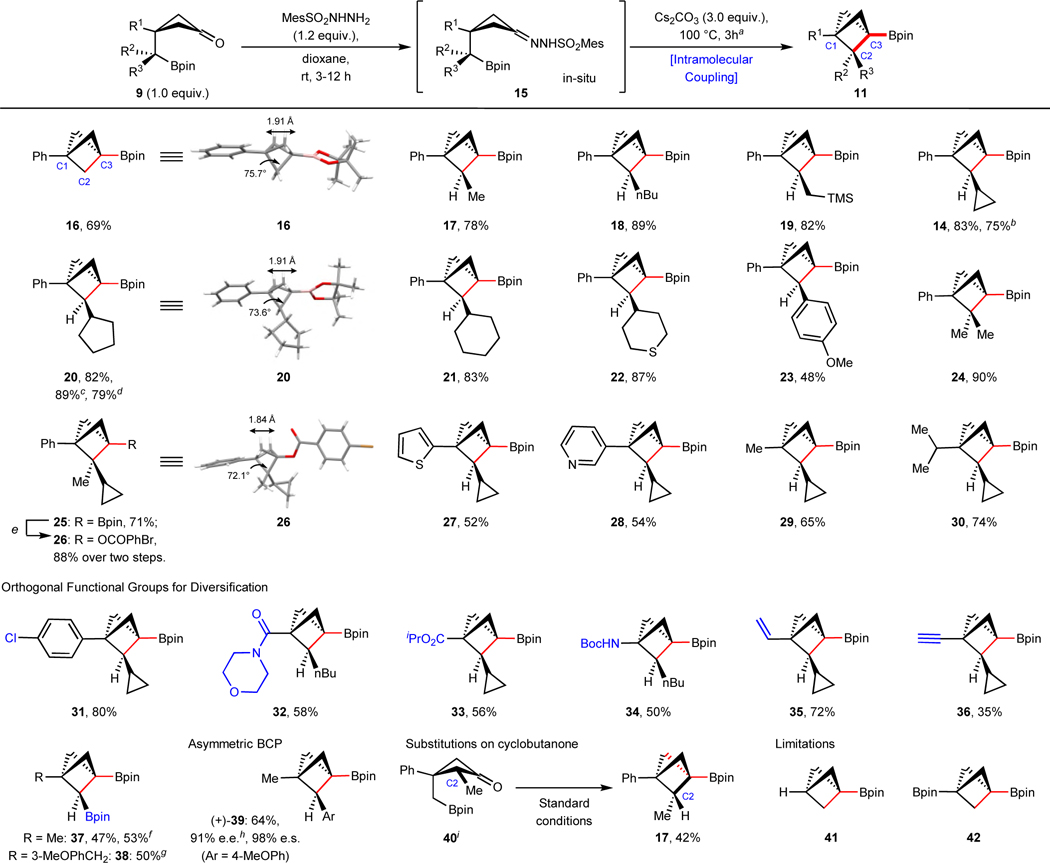
|
Starting materials and products are racemic mixtures, unless annotated.
Reaction condition: Cyclobutanone 9 (1.0 equiv., 0.05–1.0 mmol), MesSO2NHNH2 (1.2 equiv.) in dioxane (0.1–0.2 M) stirred at rt for 3–12 h, monitored by TLC; then Cs2CO3 (3.0 equiv.) was added and stirred at 100 °C for another 3 h.
4.8 mmol scale;
3.7 mmol scale;
18 mmol scale;
Reaction condition: 1) NaOAc, H2O2, 0 °C, 1 h; 2) DMAP, 4-bromobenzoyl chloride, DIPEA;
11 mmol scale;
30 mmol scale;
e.e. values were measured after conversion to their alcohol derivatives;
the stereochemistry was assigned based on its derivative; TMS, trimethylsilyl; Boc, tert-butyloxycarbonyl; DMAP, 4-dimethylaminopyridine; DIPEA, N,N-diisopropylethylamine; e.e., enantiomeric excess; e.s., enantiospecificity.
