Figure 1.
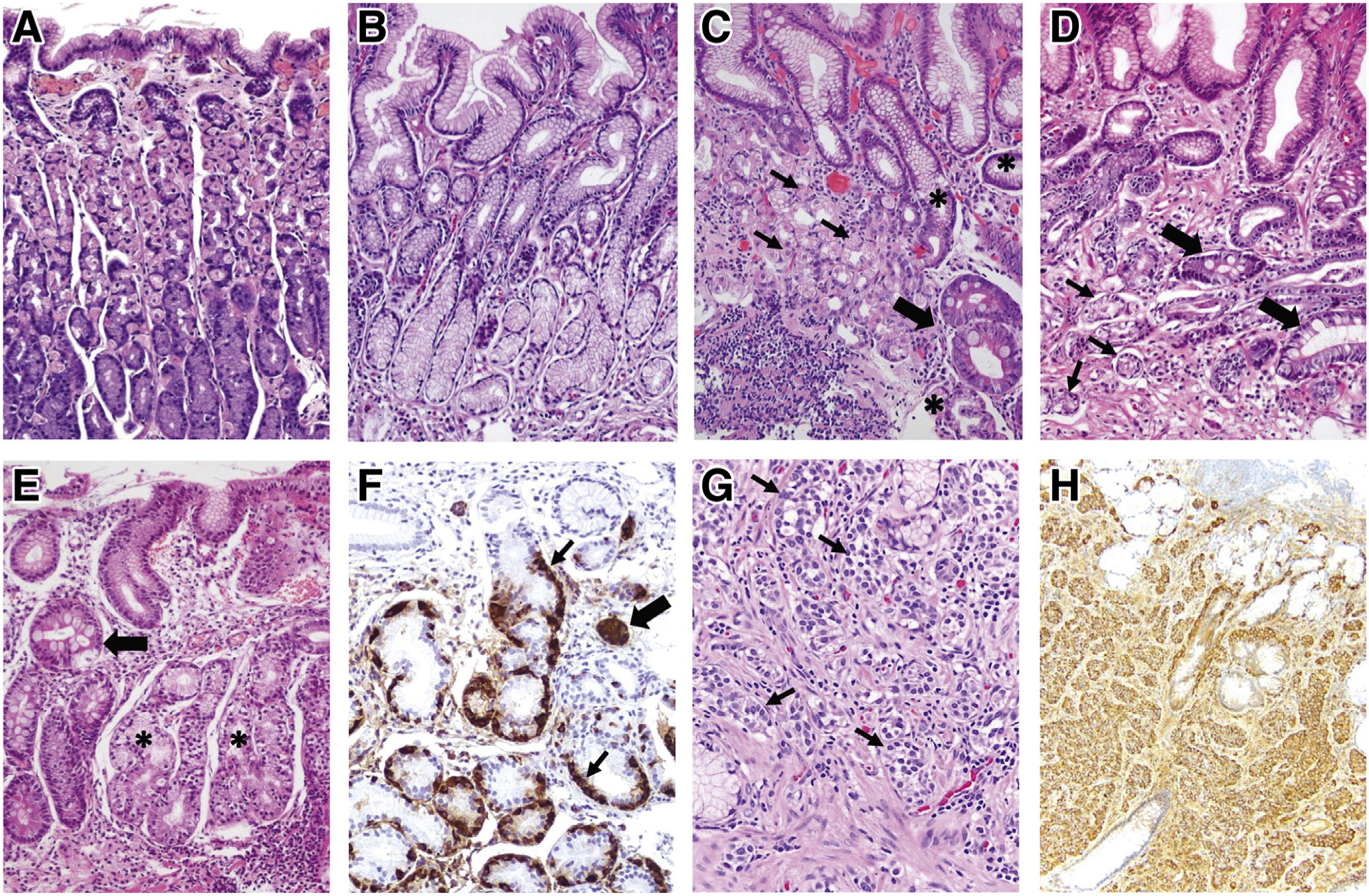
Histopathologic features of normal gastric mucosa, chronic AG, and gastric NET. (A) Normal gastric oxyntic mucosa, characterized by short foveolae (pits) and tightly packed, straight glands. The glands are primarily lined by parietal (pink) cells, which predominate in the upper two-thirds, and by chief (purple) cells at the base. (B) Normal antral mucosa, with wider foveolae and loosely packed glands, primarily lined by mucus-secreting cells. (C, D) H pylori–associated AG. (C) Corpus mucosa with chronic inflammation, moderate loss of oxyntic glands, pseudopyloric metaplasia (asterisks), and IM (thick arrow). Remaining parietal cells (thin arrows) are forming short, disorganized glands. In marked oxyntic atrophy (not shown), there may be complete absence of parietal and chief cells, making the histologic findings indistinguishable from those of antral atrophy. (D) Antral mucosa with shrunk, vanishing glands (thin arrows) and foci of IM (thick arrows) surrounded by fibromuscular tissue in the lamina propria. (E, F) Oxyntic mucosa showing fully developed autoimmune gastritis. (E) Complete absence of parietal and chief cells replaced by pseudopyloric (asterisks) and intestinal (arrow) metaplasia, in a background of chronic inflammation. In this case, glands with pseudopyloric metaplasia show ECL cell hyperplasia, highlighted in (F) with chromogranin A stain. Linear (thin arrows) and micronodular (thick arrow) ECL cell hyperplasia are observed. In earlier stages of autoimmune gastritis, the destruction of oxyntic glands by infiltrating lymphocytes (not shown) and the corpus-predominant pattern of inflammatory and atrophic changes strongly suggests this condition. (G, H) Gastric type 1 ECL cell NET. (G) The tumor is composed of well-differentiated cells with monomorphic round nuclei, arranged in small nests (arrows) infiltrating the lamina propria. The neuroendocrine differentiation is confirmed using chromogranin A stain (H).
