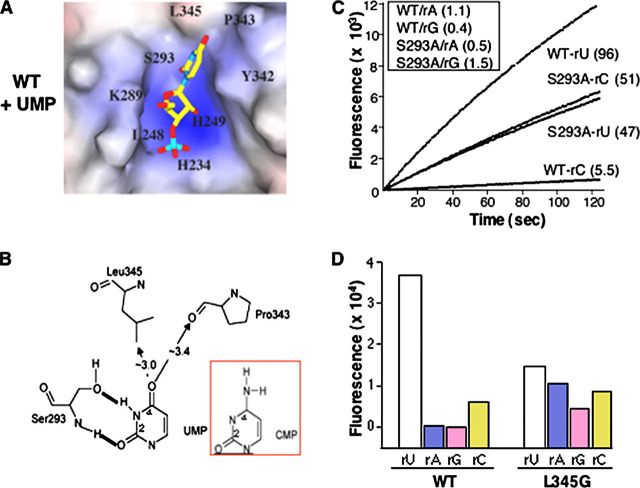
FIGURE 4.
Examination of substrate specificity.A, UMP docked into catalytic pocket of WT Nsp15. B, schematic to denote uridine recognition by S293 and the approximate distances between UMP and nearby catalytic site residues. C, results of real time fluorescent endoribonuclease assay. 0.25 μm of WT or mutant S293A proteins were used in these assays, and cleavage of substrates rU, rC, rA, and rG was measured in real time. The results of rU and rC cleavage are shown in real time. The slopes of each curve represent the rate of reaction. The identities of protein and substrate along with rate of reaction are indicated to the right of the curves. Cleavage rates of rA and rG are shown in the inset. D, cleavage of fluorescent substrates by WT or L345G proteins. Each protein (0.25 μm) was incubated with one of the four fluorogenic substrates (rU, rC, rA, or rG) for 20 min at room temperature and measured the fluorescence for each treatment. The increased cleavage of the rA substrate by L345G was confirmed in kinetic assay in two other independent experiments (data not shown).