Abstract
Background:
MK-8507 is a novel HIV-1 non-nucleoside reverse transcriptase inhibitor being developed for treatment of HIV-1 infection. MK-8507 has high antiviral potency in vitro and pharmacokinetic (PK) properties that support once-weekly dosing.
Setting:
A phase 1, open-label, proof-of-concept study was conducted in treatment-naive adults with HIV-1 infection to assess monotherapy antiviral activity.
Methods:
In 3 sequential panels, participants aged 18–60 years with baseline plasma HIV-1 RNA ≥10,000 copies/mL and CD4+ T-cell count >200/mm3 received a single oral dose of 40, 80, or 600 mg MK-8507 in the fasted state. Participants were assessed for HIV-1 RNA for at least 7 days, PKs for 14 days, and safety and tolerability for 21 days postdose.
Results:
A total of 18 participants were enrolled (6 per panel). The mean 7-day postdose HIV-1 RNA reduction ranged from ∼1.2 to ∼1.5 log10 copies/mL across the doses assessed. One patient had a viral rebound associated with emergence of an F227C reverse transcriptase variant (per chain-termination method sequencing) 14 days postdose; this variant was found in a second participant by ultra-deep sequencing as an emerging minority variant. MK-8507 PKs were generally dose-proportional and similar to observations in participants without HIV-1 infection in prior studies; mean MK-8507 half life was 56–69 hours in this study. MK-8507 was generally well tolerated at all doses.
Conclusions:
The robust antiviral activity, PK, and tolerability of MK-8507 support its continued development as part of a complete once weekly oral regimen for HIV-1 treatment; combination therapy could mitigate the emergence of resistance-associated variants.
Key Words: antiretroviral, HIV-1 infection, MK-8507, pharmacokinetics and pharmacodynamics, phase 1, treatment-naive
INTRODUCTION
Given the wide range of antiretroviral therapies available,1 people living with HIV (PLWH) have a near-normal life expectancy.2–4 Successful treatment requires strict, life-long adherence to therapy,3,5–9 which is typically administered orally as part of a once- or twice-daily regimen.10 For some PLWH, taking a daily pill is an optimal HIV treatment approach; however, for many others, less frequent administration may offer substantial benefit.11 Previous evidence suggests that adherence improves as dosing frequency decreases.12,13 Moreover, a treatment regimen that requires less frequent dosing may reduce the sense of stigma experienced by some PLWH, which is known to drive nonadherence.5,14,15 Approved HIV treatment regimens with less frequent dosing require monthly or bimonthly depot injections administered by a health care professional.16,17 Unlike long-acting depot injections, an oral once-weekly ART regimen offers PLWH the benefit of less frequent dosing with the simplicity of an oral medication. Moreover, once-weekly dosing has already been shown to improve treatment adherence across a range of chronic diseases that require long-term therapy,18,19 which may be due in part to reduced pill fatigue and a simplified dosing regimen.12
MK-8507 is a novel, oral, potent non-nucleoside reverse transcriptase inhibitor (NNRTI) in clinical development for the treatment of HIV-1 infection, that has the potential to be dosed once weekly. Preclinical studies demonstrated MK-8507 has high antiviral potency, has a half-maximal inhibitory concentration (IC50) of approximately 50 nM, and shows only modest changes in activity to the most prevalent NNRTI-associated resistance mutations (eg, K103N, Y181C).20 Clinical data in healthy subjects without HIV infection have demonstrated a pharmacokinetic (PK) profile supportive of once-weekly dosing.21 To understand the potential efficacy of MK-8507 as a once-weekly agent, this study was conducted to evaluate the antiretroviral activity of single oral doses of MK-8507 in treatment-naive PLWH. In addition, PKs, safety, and tolerability were assessed.
METHODS
Study Design
Protocol MK-8507-003 (NCT02174159) was an open-label, single-dose, proof-of-concept study conducted at Charité Research Organisation GmbH, Berlin, Germany. The study was conducted in conformance with Good Clinical Practice standards and was approved by the Ethik-Kommission des Landes Berlin, Berlin, Germany. All participants provided written informed consent before starting the study.
Eligible participants were treatment-naive male and female adults (aged 18–60 years) with HIV-1 infection. Inclusion criteria included diagnosis of HIV-1 infection ≥3 months before screening, plasma HIV-1 RNA ≥10,000 copies/mL, CD4+ T-cell count >200/mm3, no evidence of NNRTI-associated resistance mutations (eg, K103N, Y181C, and V108I) appearing in the Stanford University HIV Drug Resistance Database,22,23 and no evidence of active hepatitis C or hepatitis B infection.
The study consisted of 3 sequential panels of 6 participants each. In each panel, participants received a single dose of MK-8507 after an overnight fast: 600 mg MK-8507 in Panel A, 80 mg MK-8507 in Panel B, and 40 mg MK-8507 in Panel C. Blood samples for HIV-1 RNA were drawn at prespecified timepoints through at least 168 hours (7 days) postdose and for PK through 336 hours (14 days) postdose. Participants were followed for safety through 21 days postdose. Although not a requirement of the trial, participants were encouraged to initiate a non-NNRTI-containing HIV-1 standard-of-care (SOC) ART regimen immediately after the treatment phase of this study. The timing of SOC ART initiation was determined collaboratively between the sponsor and investigator based on the projected PK at a given dose level and the observed viral load response in preceding participants. Initiation of SOC ART was recommended at 14 days postdose for the first 3 participants at the 600 mg dose and thereafter was recommended at 7 days postdose. Safety, PKs, and HIV-1 RNA data were reviewed after each panel before dose selection and initiation of the subsequent panel.
Efficacy Measurements
Plasma samples were analyzed for HIV-1 RNA using the dual-target COBAS AmpliPrep/COBAS TaqMan HIV-1 Test (version 2.0; Roche Molecular Diagnostics, Pleasanton, CA). Samples were also analyzed for viral resistance using chain-termination method (Sanger) sequencing at screening and at the final HIV-1 RNA analysis timepoint in samples containing adequate HIV-1 RNA for sequencing (ie, minimum of 3 log10 copies/mL) using ViroSeq HIV-1 Genotyping System version 2.0 (Celera, Alameda, CA). Additional timepoints were analyzed as clinically indicated. Ultra-deep sequencing (UDS) was performed on a subset of samples to further investigate the presence of minority variants not detectable by chain-termination method sequencing. Amplicons were purified using Agencourt AMPure XP PCR purification beads on a BioMek NX workstation (Beckman Coulter, Krefeld, Germany) and quantified fluorometrically on a FluoStar Optima (BMG Labtech, Ortenberg, Germany) using Quant-iT Picogreen dsDNA reagent (Life Technologies, Darmstadt, Germany). The sequencing library was prepared with the Nextera XT DNA Sample Preparation Kit (Illumina, San Diego, CA) according to the manufacturer's description and sequenced using a MiSeq benchtop sequencer (Illumina, San Diego, CA) generating paired-end reads of 2 × 250 base pairs with a calculated coverage of approximately 100,00×.
Plasma HIV-RNA (copies/mL) measurements from participants were pooled and analyzed based on a longitudinal data analysis model containing fixed effects for treatment, time (predose and 168 hours postdose) and treatment by time interaction, and a random effect for subject. The response vector consisted of baseline and 168 hours postbaseline values. Time was treated as a categorical variable and the change from baseline for each dose level at 168 hours postbaseline was estimated. A posterior distribution for the true mean change from baseline at 168 hours was generated for each dose level using flat priors under a normal likelihood assumption. Historical placebo data from recent similarly designed monotherapy studies in treatment-naive PLWH were separately analyzed based on a longitudinal data model containing fixed effects for study and time and a random effect for subject. The change from baseline for placebo at 168 hours postdose was estimated from this model, and a posterior distribution for the true mean change from baseline at 168 hours was generated using flat priors under a normal likelihood distribution. Using the posterior distributions for each dose level and placebo, the posterior distribution of the true mean difference between each dose level and placebo was generated.
Safety
Safety was evaluated throughout the study by clinical assessment of adverse events (AEs) and repeated measurement of vital signs, physical examinations, 12-lead electrocardiograms, and laboratory safety tests (hematology, chemistry, and urinalysis).
Pharmacokinetics
MK-8507 in plasma was extracted by protein precipitation and analyzed by Merck & Co., Inc., (West Point, PA) using liquid–liquid extraction for analyte isolation followed by liquid chromatographic-tandem mass spectrometric detection. The lower limit of quantitation was 1.0 ng/mL (2.03 nM) with a linear calibration range from 1.0 to 1000 ng/mL. All PK parameter values were calculated by noncompartmental analysis using the software Phoenix WinNonlin Professional (Version 6.3; Certara, Princeton, NJ). Maximum plasma concentration (Cmax), plasma concentration at 168 hours (C168hr), and time to maximum plasma concentration (Tmax) were generated from the observed plasma concentration time data. Area under the concentration–time curve from time 0 to infinity (AUC0–∞) and time 0–168 hours (AUC0–168) were calculated using the linear trapezoidal method for ascending concentrations and the log trapezoidal method for descending concentrations (linear-up/log-down).
The PK/pharmacodynamic (PD) relationship was assessed by examining the correlation between MK-8507 C168hr and the reduction in plasma HIV-1 RNA levels from baseline at 168 hours (7 days) postdose with a nonlinear least squares approach using an Emax model. The nonlinear least squares analysis was conducted using R (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
RESULTS
Participants
In total, 18 men with HIV-1 infection participated in the trial between September 15, 2014, and July 23, 2015. Baseline demographics are summarized in Table 1.
TABLE 1.
Baseline Demographics
| MK-8507 (40 mg) | MK-8507 (80 mg) | MK-8507 (600 mg) | Total | |
| (n = 6) | (n = 6) | (n = 6) | (N = 18) | |
| Sex | ||||
| Male, n (%) | 6 (100.0) | 6 (100.0) | 6 (100.0) | 18 (100.0) |
| Age (yrs) | ||||
| Median (min, max) | 31.0 (29, 56) | 36.0 (31, 45) | 36.0 (22, 46) | 34.5 (22, 56) |
| Weight (kg) | ||||
| Mean (min, max) | 75.0 (63.9, 97.1) | 74.9 (65.5, 87.0) | 71.3 (57.9, 82.7) | 73.7 (57.9, 97.1) |
| BMI (kg/m2) | ||||
| Mean (min, max) | 22.8 (20.7, 24.8) | 23.4 (21.3, 26.6) | 21.8 (17.7, 23.2) | 22.7 (17.7, 26.6) |
| Race | ||||
| White, n (%) | 6 (100.0) | 6 (100.0) | 6 (100.0) | 18 (100.0) |
| Ethnicity | ||||
| Hispanic or Latino, n (%) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (5.6) |
| CD4+ count (cells/µL) | ||||
| Median (min, max) | 506 (253, 1069) | 494 (305, 668) | 417 (290, 571) | 468 (253, 1069) |
| Plasma HIV-1 RNA, (log10 copies/mL) | ||||
| Median (min, max) | 4.4 (4.1, 5.0) | 4.9 (4.1, 5.1) | 4.7 (4.0, 4.9) | 4.6 (4.0, 5.1) |
Antiretroviral Activity
Administration of single oral doses of MK-8507 resulted in HIV-1 RNA reductions of 1.2 log10 copies/mL at 40 mg and 1.5 log10 copies/mL at 80 and 600 mg at 168 hours (7 days) postdose (Table 2 and Fig. 1A). The first 3 participants enrolled (600 mg dose level) initiated SOC ART at 14 days postdose; 2 exhibited continued decline in HIV-1 RNA between 7 and 14 days postdose (from −1.59 to −1.72 and from −1.73 to −1.92 log10 copies/mL) and 1 exhibited HIV-1 RNA rebound with a change from baseline of −1.24 to a change of −0.8 log10 copies/mL between 7 and 14 days postdose. Subsequently enrolled participants were offered SOC ART beginning at 7 days postdose. No other participant in the trial exhibited HIV-1 viral rebound. One participant in the 40 mg dose group declined SOC ART and was followed through 14 days postdose; this participant exhibited a continued decline in HIV-1 RNA change from baseline from −1.03 to −2.10 log10 copies/mL between 7 and 14 days postdose.
TABLE 2.
Change in Plasma HIV-1 RNA From Baseline at Day 7 After Single-Dose Administration of MK-8507 in Treatment-Naive Individuals With HIV-1 Infection and Historical Data From Treatment-Naive Individuals Administered Placebo
| Historical Placebo | MK-8507 (40 mg) | MK-8507 (80 mg) | MK-8507 (600 mg) | |
| (N = 20) | (n = 6) | (n = 6) | (n = 6) | |
| LS mean | ||||
| log10 copies/mL (95% CI) | −0.03 (−0.13 to 0.08) | −1.22 (−1.52 to −0.91) | −1.50 (−1.80 to −1.19) | −1.53 (−1.84 to −1.23) |
| Posterior mean adjusted by historical placebo | ||||
| log10 copies/mL | N/A | −1.24 | −1.53 | −1.56 |
CI, confidence interval; LS, least squares.
FIGURE 1.
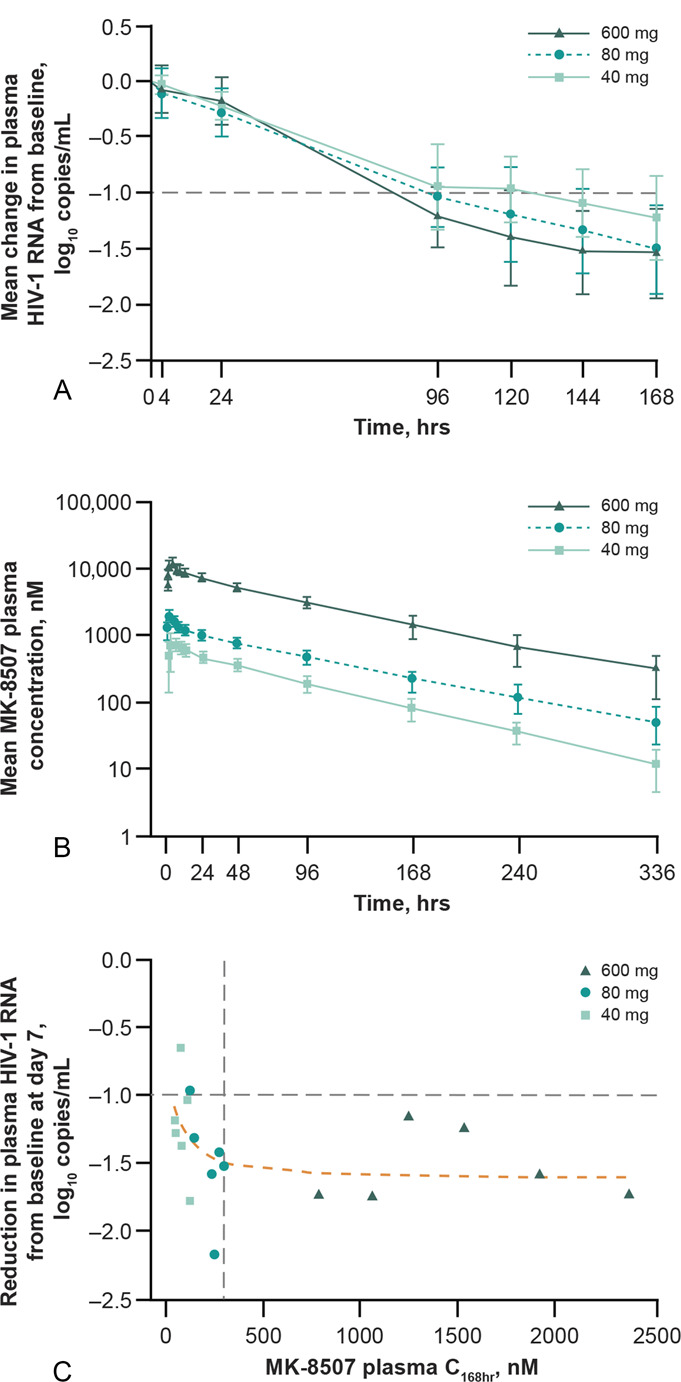
Antiviral activity and PK/PD profile of MK-8507 after a single oral dose of MK-8507 600 mg (n = 6), 80 mg (n = 6), and 40 mg (n = 6) in treatment-naive individuals with HIV-1 infection. A, Mean (SD) change in plasma HIV-1 RNA over 168 hours (7 days). B, Mean (SD) MK-8507 plasma concentration over time. C, Correlation between plasma MK-8507 C168hr and reduction in plasma HIV-1 RNA from baseline at 168 hours (7 days) postdose. Vertical dashed gray line: C168hr = 300 nM (6-fold IC50); horizontal dashed gray line: −1.0log10 reduction in HIV-1 RNA from baseline (minimum expected decline in HIV-1 RNA); dashed orange line: An NLS analysis was performed to describe the PK/PD relationship using an Emax model. C168hr, plasma concentration at 168 hours (7 days) postdose; NLS, nonlinear least squares; PD, pharmacodynamic; PK, pharmacokinetic.
Viral Resistance
Resistance testing on the final HIV-1 RNA timepoint using chain-termination method sequencing was conducted in participants with adequate HIV-1 RNA (minimum of 3 log10 copies/mL) and included 1 participant at the 600 mg dose level, 2 at the 80 mg dose level, and 4 at the 40 mg dose level (Table 3). Testing revealed the presence of F227C beginning at 10 days postdose in the 600 mg panel in the 1 participant exhibiting HIV-1 viral rebound; no other NNRTI-associated–resistance mutation was detected via chain-termination method sequencing. UDS was performed on a subset of samples following the 600 and 80 mg doses for further insight (Table 3). In the participant with HIV-1 viral rebound, F227C comprised 99% of the viral population by 14 days postdose. In another participant in the 600 mg panel exhibiting continued decline in HIV-1 RNA during the second week postdose, F227C was detected as a minority variant (21%) at 14 days postdose. Neither of these participants had F227C above the 2% threshold for reporting before dosing. In the other 4 participants receiving 600 mg MK-8507, 2 had samples that could not be amplified and 2 had no NNRTI-resistance–associated mutations detected by UDS. The E138G and V179D variants were detected in 1 participant each in the 600 mg panel before dosing, but were not detected postdose. At the 80 mg dose level, 2 participants were sequenced via UDS; in 1 participant, no NNRTI-resistance–associated mutations were found, whereas K103N (4.7%) and V189I (3.7%) were detected as minor variants in the other at the final timepoint. Results of sequencing and accompanying HIV-1 RNA levels are also shown in Table 3.
TABLE 3.
Results of Chain-Termination Method and Ultra-Deep Sequencing With Accompanying HIV-1 RNA Levels for Individual Participants
| Timepoint Relative to dosing* | HIV-1 RNA (log10 Copies/mL) | NNRTI-Resistance–Associated Mutations | |
| Chain-Termination Method Sequencing | Ultra-Deep Sequencing, Detected at >2% | ||
| MK-8507 (40 mg), h postdose | |||
| Predose, 168 | 4.46, 3.29 | ND, ND | N/A, N/A |
| Predose, 168 | 4.19, 2.41 | ND, ND† | N/A, N/A |
| Predose, 168 | 4.38, 3.73 | ND, ND | N/A, N/A |
| Predose, 168 | 4.49, 3.12 | ND, N/A | N/A, N/A |
| Predose, 168 | 4.98, 3.70 | ND, ND | N/A, N/A |
| Predose, 168 | 4.10, 3.07 | ND, ND | N/A, N/A |
| 240‡, 336‡ | 3.16, 2.00 | ND, N/A | N/A, N/A |
| MK-8507 (80 mg), h postdose | |||
| Predose, 168 | 4.97, 2.80 | ND, N/A | N/A, N/A |
| Predose, 168 | 5.13, 4.16 | ND; ND | ND, ND |
| Predose, 168 | 4.75, 3.23 | ND, N/A | N/A, N/A |
| Predose, 168 | 4.95, 3.63 | ND, ND | ND, K103N (4.7%), V189I (3.7%) |
| Predose, 168 | 4.29, 2.71 | ND, N/A | N/A, N/A |
| Predose, 168 | 4.07, 2.63 | ND, N/A | N/A, N/A |
| MK-8507 (600 mg), h postdose | |||
| Predose, 168, 336 | 4.90, 3.17, 2.98 | ND, N/A, N/A | ND, No amplification, ND |
| Predose | 4.85 | ND | V179D (25%) |
| 4, 12, 24 | 4.97, N/A, 4.81 | ND, ND, ND | N/A, N/A, N/A |
| 96, 120, 144 | 3.97, 3.85, 3.67 | ND, ND, ND | N/A, N/A, N/A |
| 168 | 3.61 | ND | ND |
| 240 | 3.40 | F227C and F227F, wild type | N/A |
| 336 | 4.45 | F227C | F227C (99%) |
| Predose | 4.00 | ND | E138G (3.4%) |
| 168 | 2.41 | N/A | No amplification |
| 336 | 2.28 | N/A | F227C (21%) |
| Predose, 168 | 4.64, 2.90 | ND, N/A | ND, ND |
| Predose, 168 | 4.18, 3.03 | ND, N/A | ND, No amplification |
| Predose, 168 | 4.79, 3.05 | ND, N/A | ND, No amplification |
Italics denotes final HIV-1 RNA timepoint and corresponding HIV-1 RNA level before SOC initiation or end of study for participant declining SOC treatment.
Sample was run although the viral RNA level did not meet the ∼1000 copy/mL threshold for chain-termination method sequencing; against expectation, the sequencing was successful and the result is reported here.
HIV-1 RNA and resistance test results are provided for 240 and 336 h postdose because this participant did not start antiretroviral therapy after 168 h.
Bolded values indicate resistance substitutions present before and after receiving a single oral dose of MK-8507, respectively.
h, hours; N/A, not analyzed, insufficient HIV-1 RNA and/or sample volume for analysis; ND, none detected NNRT, non-nucleoside reverse transcriptase inhibitor; SOC, standard of care.
Safety and Tolerability
MK-8507 was generally well tolerated and no participant discontinued the study because of an AE. Seven participants experienced a total of 10 AEs. The most common were nasopharyngitis (n = 3, 2 moderate and 1 mild) and headache (n = 3, 2 moderate and 1 mild). Of the 10 AEs, only the 3 events of headache were considered by the investigator to be related to the study drug. There were no clinically meaningful trends in vital signs, electrocardiograms, or laboratory tests. One participant who received 600 mg MK-8507 was diagnosed with diffuse large B cell lymphoma after having started SOC ART; this serious AE was not considered related to study drug and the participant recovered with sequelae. All other AEs were mild or moderate in intensity and resolved by the end of the study.
Pharmacokinetics
Plasma MK-8507 PKs are summarized in Table 4 and plasma concentration–time profiles are shown in Figure 1B. MK-8507 PKs were generally dose-proportional. The mean terminal plasma half-life of MK-8507 ranged from 56 to 69 hours across dose groups.
TABLE 4.
MK-8507 Plasma PKs After Single-Dose Administration of MK-8507 in Treatment-Naive Individuals With HIV-1 Infection
| MK-8507 (40 mg) | MK-8507 (80 mg) | MK-8507 (600 mg) | |
| (n = 6) | (n = 6) | (n = 6) | |
| GM (%GCV) | GM (%GCV) | GM (%GCV) | |
| Cmax, µM | 0.90 (23.8) | 2.11 (11.1) | 12.8 (16.8) |
| Tmax, h* | 3.00 (1.00–6.00) | 2.00 (2.00–2.00) | 4.00 (2.00–8.00) |
| Apparent terminal t1/2, h | 56.1 (16.0) | 69.4 (16.4) | 62.6 (21.3) |
| AUC0–∞, μM⋅h | 52.7 (23.1) | 129 (21.9) | 878 (20.3) |
| AUC0–168 h, μM⋅h | 45.9 (21.4) | 105 (17.8) | 740 (15.2) |
| C168 h, nM | 78.1 (38.3) | 214 (36.9) | 1400 (41.7) |
Median (range).
AUC0–∞, area under the concentration–time curve from time 0 to infinity; AUC0–168hr, area under the concentration–time curve from time 0–168 h; C168hr, plasma concentration at 168 h; Cmax, maximum plasma concentration; GCV, geometric coefficient of variation; GM, geometric mean; h, hours; PKs, pharmacokinetics; t1/2, plasma half-life; Tmax, time to maximum plasma concentration.
Pharmacokinetics/Pharmacodynamics
A scatterplot of individual weekly Ctrough (C168hr) and HIV-1 RNA change from baseline at 7 days is shown in Figure 1C. In general, most data points seem to be on the flat, maximal-effect portion of the exposure–response relationship, with a trend toward decreased response at the lower end of the C168hr range at the 40 mg dose.
DISCUSSION
MK-8507 is a novel, oral NNRTI in clinical development for the treatment of HIV-1 infection with once-weekly administration. This study demonstrated that single MK-8507 doses at 80 mg and higher achieved robust antiviral efficacy as demonstrated by the HIV-1 RNA level reductions at 7 days postdose in treatment-naive PLWH. Analysis of monotherapy studies of 9 marketed or investigational NNRTIs showed that for this class of antiretrovirals, a mean decrease in HIV-1 RNA of at least 1 log10 copies/mL should be expected after 7 days of daily dosing at a maximally effective NNRTI dose.24 This degree of reduction in HIV-1 RNA was observed with MK-8507 at all dose levels studied at 7 days postdose, indicating that single doses of MK-8507 as low as 40 mg seem comparable to the efficacy achieved with once-daily NNRTIs after 7 days of dosing. The change in HIV-1 RNA at 40 mg MK-8507, the lowest dose examined, was 1.2 log10 copies/mL; the change at both the 80 and 600 mg was 1.5 log10 copies/mL, suggesting the higher doses were on the flat, maximal portion of the exposure/response relationship as demonstrated by the regression line in Figure 1C.
Plasma Ctrough has been identified as a strong PK correlate of efficacy for once-daily NNRTIs and other classes of HIV antiretrovirals.24–27 Analysis of marketed NNRTIs found that a threshold mean Ctrough concentration that is at least 6-fold higher than the in vitro IC50 was associated with long-term efficacy in combination therapy.24 As such, antiretroviral efficacy 1 week after a single dose of MK-8507 was assessed relative to the 7-day postdose concentration (C168hr). For MK-8507, the C168hr target is ≥300 nM (ie, 6 × IC50). Doses were selected in this study to characterize the full range of the PK-viral response relationship to support use of exposure–response modeling for selection of doses for further investigation. The high dose was selected to significantly exceed the C168hr target of 300 nM at 7 days postdose to inform estimates of Emax, whereas the mid-dose was projected to achieve target viral load reductions (ie, a mean decrease in HIV-1 RNA of at least 1 log10 copies/mL) with a C168hr falling close to the PK target at 7 days postdose. The low dose was projected to achieve a half-maximal response (ie, EC50). Extrapolating between doses, the PK target of 300 nM would have been just met at a dose of 100 mg, which is the lowest dose being studied in phase 2.28 Nonetheless, antiviral activity was also observed at doses achieving lower mean C168hr values. Analysis of C168hr values versus 7-day postdose HIV-1 RNA change from baseline showed efficacy over a range of concentrations, which seems to decrease in the lower C168hr range. These data support the hypothesis that the exposure/response relationship observed for daily NNRTIs can be translated to a C168hr/efficacy relationship for a once-weekly NNRTI. However, additional data in long-term efficacy studies will be necessary to more fully characterize MK-8507 exposure–response.
MK-8507 PKs in PLWH, as assessed in this study, were generally consistent with those observed in participants without HIV infection in the single and multiple ascending dose study,21 with continued demonstration of a long half-life (geometric mean across all panels of 62 hours in this study), supportive of once-weekly administration. Minimal accumulation is anticipated with once-weekly dosing based on the PK results; therefore, the PK and PK/PD findings from this study can be readily translated to multiple dosing.
F227C is an NNRTI-resistance–associated variant that is clinically rare (≤0.5% of all clinical samples),20,29,30 observed primarily in patients receiving doravirine and less frequently in people receiving etravirine or rilpivirine.31–33 Viral rebound 14 days postdose was seen in 1 participant with F227C. In a second participant without a viral rebound 14 days postdose, F227C was found as a minority variant using UDS. This indicates that a viral rebound may have occurred had ART not been started. Consequently, UDS could be a valuable tool in clinical research to detect the emergence of resistance mutations before an upcoming viral rebound and prior to their detection through resistance testing with standard chain-termination method sequencing. Two NNRTI-resistance–associated variants (E138G and V179D) were detected in 1 participant each in the 600 mg panel before dosing, but were not detected postdose, indicating that MK-8507 did not select for these. At the 80 mg dose level, UDS but not the chain-termination method detected K103N and V189I at <5% at 7 days postdose, although viral load showed continued decline at this timepoint. Sequencing at other timepoints would be required to better understand their significance. Although UDS provides in-depth insight into low-level variants, these results point to the importance of analyzing multiple samples and/or timepoints before drawing conclusions about their significance.
As resistance to HIV antiretrovirals typically emerges rapidly, the standard approach to therapy is use of at least 2 different classes of drugs for combination ART.10,34,35 No rebound was observed in the first 7 days postdose in this study, and detection of K103N at 7 days postdose in 1 participant and F227C in another at 14 days postdose were not accompanied by rebound. F227C would not be expected to emerge in combination with an antiretroviral of another class with activity against F227C; the intended clinical use of MK-8507 is as part of a weekly combination therapy. The virologic profile of MK-8507 will be further assessed in longer and larger controlled clinical trials of MK-8507.28
The main limitations to consider when interpreting the findings are the short study length and limited population, without any women enrolled. Further studies will serve to establish the efficacy and safety of MK-8507, in a wider population of PLWH over a longer period of time, as part of combination ART. Currently available antiretroviral regimens require at least once-daily administration, or in the case of cabotegravir/rilpivirine, a monthly or bimonthly intramuscular injection at a clinic. Pending results of further studies, MK-8507 has the potential to be part of combination ART as a once-weekly regimen with the ease of at-home oral dosing, without the daily reminder of HIV infection associated with daily dosing. Together, the antiviral efficacy, safety and tolerability, and PK findings support the potential to administer MK-8507 as part of a complete once-weekly oral regimen for the treatment of HIV-1 infection.
ACKNOWLEDGMENTS
The authors thank the participants in this study, Brad Roadcap (Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ) for oversight of the bioanalytical work, and Robin Mogg (Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ) for statistical expertise. The contributions of the investigators and their staff are also gratefully recognized, and Anke Schulz, Susanne Schäffer, and Andreas Hüser (Charité Research Organisation GmbH, Berlin, Germany) are acknowledged for their contributions to the study. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship. Medical writing and editorial assistance, under the direction of the authors, were provided by Jessica Wong of ApotheCom (UK) in accordance with Good Publication Practice (GPP3) guidelines.
Footnotes
Y.L., B.K., D.J.R., A.S., I.D.L., E.J.F., M.R., S.Z., J.A.G., S.A.S., M.I., and W.A. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) and may own stock in Merck & Co., Inc., Kenilworth, NJ, USA. M.D. has received consultancy fees from MSD. Funding for this research was provided by MSD. Medical writing and editorial assistance were provided by ApotheCom and was funded by MSD.
Meetings at which parts of the data were presented: HIV Drug Therapy Glasgow 2020; October 5-8, 2020 (virtual) and Conference on Retroviruses and Opportunistic Infections 2021, March 6-10, 2021 (virtual).
Contributor Information
Dirk Schürmann, Email: dirk.schuermann@charite.de.
Deanne Jackson Rudd, Email: deanne_rudd@merck.com.
Andrea Schaeffer, Email: andrea_schaeffer@merck.com.
Inge De Lepeleire, Email: inge_de_lepeleire@merck.com.
Evan J. Friedman, Email: evan.jeffrey.friedman@gmail.com.
Martine Robberechts, Email: martinerobberechts@hotmail.com.
Saijuan Zhang, Email: saijuanzhang@gmail.com.
Yang Liu, Email: yang_liu2@merck.com.
Bhargava Kandala, Email: bhargava.kandala@merck.com.
Christian Keicher, Email: christian.keicher@charite-research.org.
Martin Däumer, Email: m.daeumer@immungenetik-kl.de.
Jörg Hofmann, Email: joerg.hofmann@charite.de.
Jay A. Grobler, Email: jay_grobler@merck.com.
S. Aubrey Stoch, Email: aubrey_stoch@merck.com.
Marian Iwamoto, Email: marian.iwamoto@merck.com.
REFERENCES
- 1.AIDSMAP. Types of antiretrovial medications. 2020. Available at: https://www.aidsmap.com/about-hiv/types-antiretroviral-medications. Accessed April 1, 2021.
- 2.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith DE, Woolley IJ, Russell DB, et al. HIV in practice: current approaches and challenges in the diagnosis, treatment and management of HIV infection in Australia. HIV Med. 2018;19(suppl 3):5–23. [DOI] [PubMed] [Google Scholar]
- 4.Marcus JL, Leyden WA, Alexeeff SE, et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection. JAMA Netw Open. 2020;3:e207954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iacob SA, Iacob DG, Jugulete G. Improving the adherence to anti-retroviral. therapy, a difficult but essential task for a successful HIV treatment-clinical points of view and practical considerations. Front Pharmacol. 2017;8:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tseng A, Seet J, Phillips EJ. The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future. Br J Clin Pharmacol. 2015;79:182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. [DOI] [PubMed] [Google Scholar]
- 8.Harrigan PR, Hogg RS, Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191:339–347. [DOI] [PubMed] [Google Scholar]
- 9.Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–1058. [DOI] [PubMed] [Google Scholar]
- 10.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. 2019. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. Accessed June 1, 2021. [Google Scholar]
- 11.Solomon DA, Sax PE. Current state and limitations of daily oral therapy for treatment. Curr Opin HIV AIDS. 2015;10:219–225. [DOI] [PubMed] [Google Scholar]
- 12.Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. [DOI] [PubMed] [Google Scholar]
- 14.Rintamaki L, Kosenko K, Hogan T, et al. The role of stigma management in HIV treatment adherence. Int J Environ Res Public Health. 2019;16:5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. Plos Med. 2016;13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabenuva. Prescribing information. Viiv Healthcare; 2021. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212888s000lbl.pdf. Accessed November 2, 2021. [Google Scholar]
- 17.European Medicines Agency. Assessment Report: Vocabria. Published October 15, 2020. Available at: https://www.ema.europa.eu/en/documents/assessment-report/vocabria-epar-public-assessment-report_en.pdf. Accessed February 17, 2021. [Google Scholar]
- 18.Iglay K, Cao X, Mavros P, et al. Systematic literature review and meta-analysis of medication adherence with once-weekly versus once-daily therapy. Clin Ther. 2015;37:1813–1821.e1. [DOI] [PubMed] [Google Scholar]
- 19.Qiao Q, Ouwens MJ, Grandy S, et al. Adherence to GLP-1 receptor agonist therapy administered by once-daily or once-weekly injection in patients with type 2 diabetes in Germany. Diabetes Metab Syndr Obes. 2016;9:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamond TL, Lai MT, Feng M, et al. Resistance profile of MK-8507, a novel NNRTI suitable for weekly oral HIV treatment. Presented at the Conference on Retroviruses and Opportunistic Infections (CROI) 2021 (virtual); March 6–10, 2021. Abstract #129.
- 21.Ankrom W, Schürmann D, Jackson Rudd D, et al. Oral presentation: Single doses of MK-8507, a novel HIV-1 NNRTI, reduced HIV viral load for at least a week. HIV Glasgow 2020; October 5–8, 2020 (Virtual).
- 22.Stanford University. Stanford University HIV Drug Resistance Database: HIVdb Program. Available at: https://hivdb.stanford.edu/hivdb/by-mutations/. Accessed August 13, 2020. [Google Scholar]
- 23.Shafer RW, Jung DR, Betts BJ. Human immunodeficiency virus type 1 reverse transcriptase and protease mutation search engine for queries. Nat Med. 2000;6:1290–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Li YF, Zhang D, et al. Characterizing class-specific exposure-viral load suppression response of HIV antiretrovirals using a model-based meta-analysis. Clin Transl Sci. 2016;9:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acosta EP, Limoli KL, Trinh L, et al. Novel method to assess antiretroviral target trough concentrations using in vitro susceptibility data. Antimicrob Agents Chemother. 2012;56:5938–5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preston SL, Piliero PJ, Bilello JA, et al. In vitro-in vivo model for evaluating the antiviral activity of amprenavir in combination with ritonavir administered at 600 and 100 milligrams, respectively, every 12 hours. Antimicrob Agents Chemother. 2003;47:3393–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizk ML, Hang Y, Luo WL, et al. Pharmacokinetics and pharmacodynamics of once-daily versus twice-daily raltegravir in treatment-naive HIV-infected patients. Antimicrob Agents Chemother. 2012;56:3101–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov. NCT04564547: Dose Ranging, Switch Study of Islatravir (ISL) and MK-8507 Once-Weekly in Virologically-Suppressed Adults With Human Immunodeficiency Virus Type 1 (HIV-1) [MK-8591-013]. Available at: https://clinicaltrials.gov/ct2/show/NCT04564547. Accessed August 5, 2020. [Google Scholar]
- 29.Soulie C, Santoro MM, Storto A, et al. Prevalence of doravirine-associated resistance mutations in HIV-1-infected antiretroviral-experienced patients from two large databases in France and Italy. J Antimicrob Chemother. 2020;75:1026–1030. [DOI] [PubMed] [Google Scholar]
- 30.Calvez V, Marcelin AG, Vingerhoets J, et al. Systematic review to determine the prevalence of transmitted drug resistance mutations to rilpivirine in HIV-infected treatment-naive persons. Antivir Ther. 2016;21:405–412. [DOI] [PubMed] [Google Scholar]
- 31.Rimsky L, Vingerhoets J, Van Eygen V, et al. Genotypic and phenotypic characterization of HIV-1 isolates obtained from patients on rilpivirine therapy experiencing virologic failure in the phase 3 ECHO and THRIVE studies: 48-week analysis. J Acquir Immune Defic Syndr. 2012;59:39–46. [DOI] [PubMed] [Google Scholar]
- 32.Orkin C, Squires K, Molina J, et al. Doravirine/lamivudine/tenofovir disoproxil fumarate is non-inferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive adults with human immunodeficiency virus-1 infection: week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis. 2018;68:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanford University. Major Non-Nucleoside RT Inhibitor (NNRTI) Resistance Mutations. 2021. Available at: https://hivdb.stanford.edu/dr-summary/resistance-notes/NNRTI/. Accessed August 13, 2021.
- 34.European AIDS Clinical Society (EACS). European Guidelines for the Treatment of HIV-Positive Adults in Europe Version 10.1. 2020. Available at: https://www.eacsociety.org/files/guidelines-10.1.finalsept2020.pdf. Accessed April 1, 2021. [Google Scholar]
- 35.Pennings PS. HIV drug resistance: problems and perspectives. Infect Dis Rep. 2013;5(suppl 1):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]


