Abstract
We report on a case of mixed infection caused by two species of Fusarium in a human immunodeficiency virus-positive patient with lymphoma who was neutropenic due to chemotherapy. The patient showed the typical signs of a disseminated fusarial infection, with Fusarium solani isolated from skin lesions and F. verticillioides isolated from blood. The report discusses how difficult it is to make an accurate diagnosis when an immunosuppressed patient is infected with more than one fungal species, especially when the species are morphologically very similar.
Disseminated fusarial infections are associated with a high rate of mortality. Their treatment constitutes a difficult challenge for modern medicine. Approximately eight species of Fusarium are recognized as opportunistic pathogens that cause disseminated infections in the compromised host (6). The recovery of two Fusarium species from the same patient is unusual and warrants special attention by clinical microbiologists. This report describes a fatal case of fusarial infection in a neutropenic human immunodeficiency virus (HIV)-positive patient with lymphoma from whom Fusarium solani and F. verticillioides, the two most common species of this genus pathogenic for humans, were isolated. To our knowledge, this is the first published report of simultaneous infection with two species of Fusarium.
Case report.
A 50-year-old Brazilian man was admitted to the Hospital Universitário Clementino Fraga Filho because of fever and neutropenia after chemotherapy for high-grade non-Hodgkin's lymphoma. He had been severely neutropenic for 11 days and had received ciprofloxacin, amikacin, fluconazole, and granulocyte colony-stimulating factors (G-CSF). He was seropositive for HIV. Physical examination revealed pallor, mild jaundice, moderate mucositis, and fever (axillary temperature, 37.8°C). The patient's hemoglobin concentration was 11.2 g/dl, the leukocyte count was 600/mm3 with 150 neutrophils/mm3, and the platelet count was 8,000/mm3. Two blood samples were collected for culture, G-CSF was continued, and ceftazidime, amikacin, and vancomycin were administered. Two days later empirical amphotericin B (0.5 mg/kg of body weight daily) and metronidazole were added because of persistent fever and worsening of the mucositis. Two days later numerous erythematous lesions measuring 1 to 2 cm appeared on the arms, abdomen, and right leg. The patient complained of myalgia. A biopsy sample of one of the skin lesions was obtained for histopathologic examination and culture. In addition, another two blood samples were taken and the amphotericin B dosage was increased to 1 mg/kg daily. Blood samples were processed as follows. Each blood sample was inoculated into two bottles containing brain heart infusion broth, and the bottles were examined daily for at least 4 weeks. The bottles were incubated at 37°C, and blind subcultures were made after 6 to 24 h of incubation or whenever examination of the bottles suggested growth of microorganisms. The subcultures were made by taking 0.5 ml of the liquid from the bottle with disposable syringes and inoculating it into four tubes containing solid media: brain heart infusion agar (Merck KgaA, Darmstadt, Germany), Sabouraud dextrose agar (Difco Laboratories, Detroit, Mich.), mycobiotic agar (Difco), and niger seed agar. The tubes were incubated at room temperature. The fragments of the skin biopsy specimen were sliced, inoculated into four tubes containing the same solid media used for blood, and also incubated at room temperature. Numerous white colonies of a Fusarium sp. grew from all blood samples and from the biopsied tissue. However, in the histologic study with hematoxylin and eosin, Giemsa, and Gomori methenamine silver stains, there was only mild inflammation with mononuclear cells in the dermis, and no fungal elements were detected. The dosage of amphotericin B was increased to 1.5 mg/kg daily. Five days later the patient recovered from the neutropenia and the skin lesions were a little better. Two days later, the patient suddenly lost consciousness, developed respiratory and circulatory arrest, and died. An autopsy was refused.
Because all colonies were apparently identical, only two isolates were kept for the identification of the Fusarium species, one from the biopsy tissue cultures and the other from the blood cultures. For that purpose, the subcultures of both isolates were referred to the Microbiology Unit of the Faculty of Medicine at the Rovira i Virgili University in Reus, Spain.
Diagnosis of mycological infection.
The isolate from the biopsy material was designated FMR 6323 and that from blood was designated FMR 6488. Both were subcultured on potato dextrose agar (PDA; Difco) and oatmeal agar (OMA; 30 g of oat flakes, 1 g of SO4Mg · 7H2O, 1.5 g of PO4H2K, 15 g of agar, 1,000 ml of tap water; homemade) and incubated in the darkness at ca. 25°C.
Colonies of FMR 6323 on PDA attained diameters of 35 to 36 mm after 4 days, with a greyish white, dense, and floccose aerial mycelium and a bluish brown reverse. Colonies grown on OMA showed a growth rate similar to those of colonies grown on PDA, but the aerial mycelium was white and sparse. Macroconidia were abundantly produced after 7 days on both media from confluent, cream-colored sporodochia. These were hyaline, usually moderately curved, three-septate, and 30 to 43 μm long by 4 to 5.5 μm wide. Microconidia were also abundantly produced and disposed in slimy heads on monophialides. These were also hyaline and usually oval and nonseptate, measuring 7 to 15 μm by 2 to 4.5 μm. Chlamydospores were also produced, both singly and in pairs. From these features, the isolate was identified as F. solani (Fig. 1). Colonies of FMR 6488 developed more rapidly than those of FMR 6323 on PDA and OMA and reached diameters of 47 and 55 mm, respectively, after 4 days. On PDA, the aerial mycelium was first white and cottony but soon became white and vinaceous. Colonies on OMA were also vinaceous, but these were powdery due to the abundant production of microconidia in chains. Microconidia were usually clavate with a slightly truncate base, were 5 to 10 μm long by 2 to 3 μm wide, and were produced only from monophialides. Macroconidia and chlamydospores were not produced. These typical colonies and the microscopic features of the microconidia identified the isolate as F. verticillioides (F. moniliforme) (Fig. 2). Both names are widely used in the medical literature, although in recent molecular studies it has been proved that F. moniliforme is a synonym of F. verticillioides, and the latter name has priority over the former (16). F. solani and F. verticillioides can easily be differentiated mainly by the arrangement of the microconidia on the conidiogenous cells and the colony features after at least 5 days of incubation (6). Both are the most common species in disseminated infections (2, 6).
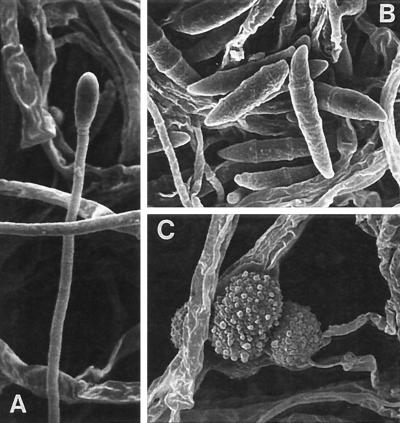
FIG. 1.
F. solani FMR 6323. (A) Phialide. Magnification by scanning electron microscopy, ×1,870. (B) Macroconidia. Magnification by scanning electron microscopy, ×1,650. (C) Chlamydospores. Magnification by scanning electron microscopy, ×2,500.
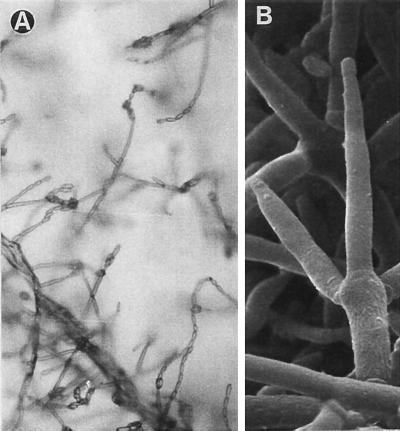
FIG. 2.
F. verticillioides FMR 6488. (A) Chains of microconidia. Magnification, ×160. (B) Conidiophore. Magnification by scanning electron microscopy, ×2,320.
The case described here was typical of an invasive fusarial infection, i.e., fever refractory to antibacterial drugs, numerous skin lesions, and myalgias (2). However, this is the first patient from whom two different species of Fusarium have been isolated. Neither of the two most commonly described portals of entry for the infection (skin and airways) (7) was evident in our patient.
Mixed fungal infections are uncommon (3, 11, 14, 17, 20, 22). Fusarium species have also been involved in concomitant infections, especially in immunocompromised patients, but with species of other genera (10, 21). When mixed infections are caused by fungi from different species, it is relatively easy to determine that more than one species is involved. However, the colonies produced by the different species of Fusarium are often very similar, especially during the first few days of incubation (Fig. 3), and they are difficult to differentiate when they are present in the same sample. Given that it can be difficult to discriminate between Fusarium colony types at an early stage of growth, it was perhaps fortuitous that one colony of F. solani was subcultured from the primary culture of the skin biopsy specimen and one colony of F. verticillioides was subcultured from the primary blood culture. Both subcultures were referred to the Microbiology Unit as two isolates of the same strain of Fusarium sp.; however, when we examined both fungi we noticed that they belonged to different species. Unfortunately, we could not obtain more samples because the patient had died. In this case no fungal structures were observed in the histological study of the biopsied material. Although the observation of hyphal elements in tissue is very important in the diagnosis of fungal infections, they are not always detected in disseminated fusarial infections (13). Additionally, we must remember that the histological appearance of Fusarium species is variable and can mimic that of several other opportunistic molds. Therefore, even when fungal structures compatible with species of Fusarium are observed, cultures are required to confirm the diagnosis and for species identification. This is important because different virulences among species have clearly been demonstrated in Fusarium. Using a murine model, we proved that F. solani was clearly more pathogenic than the other species involved in disseminated infections, i.e., F. oxysporum, F. verticillioides, and F. proliferatum (12), and moreover, F. solani is the most common species causing fatal infections in humans (7). Detection of only one species when two or more are present may result in false hope and erroneous treatment. The species detected may also be less virulent than the other one(s) present. For our patient, knowing that two species were involved would not have affected the outcome for the patient because the few antifungal drugs available are not efficacious against Fusarium either in vitro (19) or in vivo (8). However, this information could be extremely useful in other cases, for instance, in Scedosporium infections (Scedosporium prolificans and S. apiospermum show very different susceptibilities to antifungal agents) (J. Guarro, J. Cano, J. Gené, M. Solé, and A. J. Carrillo, Abstr. 14th Int. Soc. Hum. Anim. Mycol. Congr., p. 84, 2000) and in Paecilomyces infections (the two most common Paecilomyces species, Paecilomyces variotii and P. lilacinus, also show very different antifungal susceptibilities) (1).
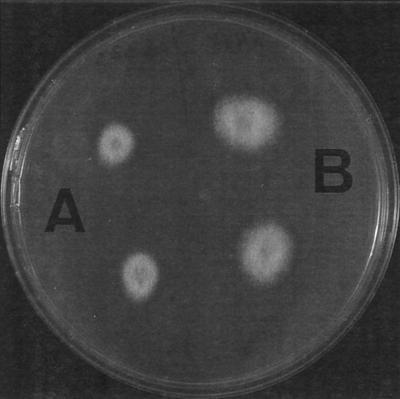
FIG. 3.
Colonies of F. solani (A) and F. verticillioides (B) on PDA at 25°C after 2 days of incubation.
In HIV-infected patients the typical opportunistic fungi such as Candida albicans, Cryptococcus neoformans, Histoplasma capsulatum, and Coccidioides immitis are found regularly, as are other, less common ones like Sporothrix schenckii and Penicillium marneffei (18). However, Fusarium species which are isolated in increasing number from immunosuppressed hosts (mainly leukemic patients) are rarely found in HIV-positive or AIDS patients. The only previously reported cases were localized infections that affect the lung (9), heart (E. Stool, J. Gathe, Jr., D. Piot, G. del Junco, and T. Anderson, 7th Int. Conf. AIDS, abstr. MB 2390, 1991), and eyes (5), although they may also have caused onychomycosis in these patients (4). To our knowledge, this is the first case of disseminated fusarial infection in an HIV-positive patient. In addition, the patient was diagnosed as having a lymphoma and was neutropenic due to chemotherapy, a setting very similar to those for the majority of patients with disseminated fusariosis reported so far (15). We therefore think that neutropenia induced by chemotherapy rather than the immunosuppression due to HIV infection played a major role in the development of this infection.
The case described here illustrates the importance of culture and of the accurate identification of the isolates, especially in immunosuppressed patients, when more than one species may be present. Medical microbiologists should be aware of this possibility.
REFERENCES
- 1.Aguilar C, Pujol I, Sala J, Guarro J. Antifungal susceptibilities of Paecilomyces species. Antimicrob Agents Chemother. 1998;42:1601–1604. doi: 10.1128/aac.42.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutati E I, Anaissie E J. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood. 1997;90:999–1008. [PubMed] [Google Scholar]
- 3.Cimerman M, Gunde-Cimerman N, Zalar P, Perkovic T. Femur osteomyelitis due to a mixed fungal infection in a previously healthy man. J Clin Microbiol. 1999;37:1532–1535. doi: 10.1128/jcm.37.5.1532-1535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cribier B, Mena M L, Rey D, Partisani M, Fabien V, Lang J M, Grosshans E. Nail changes in patients infected with human immunodeficiency virus. A prospective controlled study. Arch Dermatol. 1998;134:1216–1220. doi: 10.1001/archderm.134.10.1216. [DOI] [PubMed] [Google Scholar]
- 5.Glasgow B J, Engstrom R F, Jr, Holland G N, Kreiger A E, Wool M G. Bilateral endogenous Fusarium endophthalmitis associated with acquired immunodeficiency syndrome. Arch Ophthalmol. 1996;114:873–875. doi: 10.1001/archopht.1996.01100140087017. [DOI] [PubMed] [Google Scholar]
- 6.Guarro J, Gené J. Fusarium infections. Criteria for the identification of the responsible species. Mycoses. 1992;35:109–114. doi: 10.1111/j.1439-0507.1992.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 7.Guarro J, Gené J. Opportunistic fusarial infections in humans. Eur J Clin Microbiol Infect Dis. 1995;14:741–754. doi: 10.1007/BF01690988. [DOI] [PubMed] [Google Scholar]
- 8.Guarro J, Pujol I, Mayayo E. In vitro and in vivo experimental activities of antifungal agents against Fusarium solani. Antimicrob Agents Chemother. 1999;43:1256–1257. doi: 10.1128/aac.43.5.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernanz P A, Casado V A, López F P, Quiros H, Palancar M P. Opportunistic lung infection due to Fusarium moniliforme in an AIDS patient. Rev Iber Micol. 1989;6:144–147. [Google Scholar]
- 10.Lopes J O, de Mello E S, Klock C. Mixed intranasal infection caused by Fusarium solani and a zygomycete in a leukaemic patient. Mycoses. 1995;38:281–284. doi: 10.1111/j.1439-0507.1995.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 11.Marriot D E, Wong K H, Aznar E, Hakness J L, Cooper D A, Muir D. Scytalidium dimidiatum and Lecythophora hoffmannii: unusual causes of fungal infection in a patient with AIDS. J Clin Microbiol. 1997;35:2949–2952. doi: 10.1128/jcm.35.11.2949-2952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayayo E, Pujol I, Guarro J. Experimental pathogenicity of four opportunist Fusarium species in a murine model. J Med Microbiol. 1999;48:363–366. doi: 10.1099/00222615-48-4-363. [DOI] [PubMed] [Google Scholar]
- 13.Merz W G, Karp J E, Hoagland M, Jett-Goheen M, Junkins J M, Hood A F. Diagnosis and successful treatment of fusariosis in the compromised host. J Infect Dis. 1988;158:1046–1055. doi: 10.1093/infdis/158.5.1046. [DOI] [PubMed] [Google Scholar]
- 14.Nenoff P, Friedrich T, Schwenke H, Mierzwa M, Horn L C, Haustein U F. Rare fatal simultaneous mould infection of the lung caused by Aspergillus flavus and the basidiomycete Coprinus sp. in a leukemic patient. J Med Vet Mycol. 1997;35:65–69. [PubMed] [Google Scholar]
- 15.Nucci M, Spector N, Lucena S, Bacha P C, Pulcheri W, Lamosa A, Derossi A, Caiuby M J, Maceira J, Oliveira H P. Three cases of Fusarium species in neutropenic patients. Eur J Clin Microbiol Infect Dis. 1992;11:1160–1162. doi: 10.1007/BF01961136. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell K, Cigelnik E, Nirenberg H I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia. 1998;90:465–493. [Google Scholar]
- 17.Pec J, Palencarova E, Plank L, Straka S, Pec M, Jesenka Z, Filo V. Phaeohypomycosis due to Alternaria spp. and Phaeosclera dematioides: a histopathological study. Mycoses. 1995;39:217–221. doi: 10.1111/j.1439-0507.1996.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 18.Perfect J R, Schell W A, Rinaldi M G. Uncommon invasive fungal pathogens in the acquired immunodeficiency syndrome. J Med Vet Mycol. 1993;31:175–179. doi: 10.1080/02681219380000211. [DOI] [PubMed] [Google Scholar]
- 19.Pujol I, Guarro J, Gené J, Sala J. In vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J Antimicrob Chemother. 1997;39:163–167. doi: 10.1093/jac/39.2.163. [DOI] [PubMed] [Google Scholar]
- 20.Schulze H, Aptroot A, Grote-Metke A, Balleisten L. Aspergillus fumigatus und Chaetomium homopilatum bei einem Leukämiepatienten. Pathogene Bedeutung Chaetomium-Arters. Mycoses. 1997;40(Suppl. 1):104–109. doi: 10.1111/j.1439-0507.1997.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 21.Vasiloudes P, Morelli J G, Weston W L. Painful skin papules caused by concomitant Acremonium and Fusarium in a neutropenic patient. J Am Acad Dermatol. 1997;37:1006–1008. doi: 10.1016/s0190-9622(97)70087-2. [DOI] [PubMed] [Google Scholar]
- 22.Viallard J F, Monlun E, Neau D, Vital C, Long-Boursier M, Lebras M. Pneumonia due to Fonsecaea pedrosoi and cerebral abscesses due to Emericella nidulans in a bone marrow transplant recipient. Clin Infect Dis. 1995;21:1346–1348. doi: 10.1093/clinids/21.5.1346. [DOI] [PubMed] [Google Scholar]