Abstract
OBJECTIVE
An estimated 37 million Americans have chronic kidney disease (CKD). Nearly 90% do not know about their condition because of low awareness about the importance of CKD testing and diagnosis among practitioners and people at risk for CKD. This study uses data from a national clinical laboratory to identify guideline-recommended CKD testing rates across the U.S.
RESEARCH DESIGN AND METHODS
Patients with Laboratory Corporation of America Holdings (Labcorp) testing between 2013 and 2019 were defined as at risk for CKD if they had any testing ordered with diagnosis codes for diabetes and/or hypertension. Guideline-concordant CKD assessment was defined by estimated glomerular filtration rate (eGFR) and urine albumin-to-creatinine ratio (uACR) testing within the study year.
RESULTS
We identified 28,295,982 at-risk patients (mean age 60.6 ± 14.8 years; 53.6% women): 16.2% had diabetes, 63.8% had hypertension, and 20.1% had both comorbidities. Of these, 80.3% did not receive guideline-concordant assessment during the study period. Furthermore, only 21.0% had uACR testing versus 89.6% with eGFR. CKD assessment occurred at least once in 28.7% of patients with diabetes, 10.5% of patients with hypertension, and 41.4% of patients with both conditions. In a state-by-state comparison, annual testing rates ranged from 5 to 30%. The nationwide rate increased modestly each year between 2013 and 2018 (from 10.7% to 15.2%).
CONCLUSIONS
Despite guideline recommendations, testing for CKD with uACR and eGFR in U.S. adults with diabetes and hypertension is low in routine clinical care. These data highlight the need for strategies to improve routine CKD assessment nationwide.
Introduction
An estimated 37 million U.S. adults have chronic kidney disease (CKD) and are at risk for kidney failure or end-stage kidney disease (ESKD) requiring dialysis or kidney transplant, in addition to cardiovascular events and death. In the 2015–2016 National Health and Nutrition Examination Survey, prevalence of CKD stages G1–4 was 14.2% among adult participants (1). One in three U.S. adults is at risk for diabetes and/or high blood pressure (2). The incidence of CKD is projected to increase over the next 20 years because of increasing obesity rates and an aging U.S. population (3). Globally, increasing prevalence of type 2 diabetes is the primary causative factor accounting for increased ESKD (4).
In addition to high prevalence, the health care cost burden of CKD is substantial. Total 2016 Medicare expenditures for kidney disease were >$114 billion, totaling $79 billion for all CKD stages and $35 billion for ESKD, including dialysis and kidney transplants (5). The following year, costs increased to $84 billion for CKD without dialysis treatment and $36 billion for ESKD (6). While beneficiaries ≥65 years of age with CKD represented 14% of the Medicare population, CKD costs accounted for 25% of Medicare expenditures (6), demonstrating disproportionate health care costs for patients with kidney disease.
Despite prevalence and burgeoning costs, patient awareness remains low (7). This may be partially attributed to underdetection, even among high-risk groups (8). A 2017 state-level survey showed awareness among individuals with laboratory evidence of CKD to be 9.0% (9). Similarly, 2017 Centers for Disease Control and Prevention (CDC) data showed 90% of patients in stage G3 and 48% in G4 CKD were not aware (10). Over half of the patients who initiated dialysis in 2017 had <12 months of nephrology services, contributing to unplanned crash dialysis starts, demonstrated by 80% of patients with incident hemodialysis starting with a dialysis catheter rather than the generally preferred arteriovenous fistula or graft (6,11).
Early-stage CKD is often asymptomatic, making laboratory testing imperative for at-risk patients. Diagnosis uses two widely available, inexpensive tests recommended by Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines: 1) serum creatinine with estimated glomerular filtration rate (eGFR), a test of kidney function, and 2) urine albumin-to-creatinine ratio (uACR), a test of kidney damage (12–14). While eGFR results are common within metabolic panels, the test alone is insufficient for CKD detection: albuminuria reflects endothelial inflammation that may cause proximal tubular damage and progressive function loss (15). Both tests should be performed at least annually for at-risk patients by primary care practitioners (PCPs) and clinicians who manage diabetes and hypertension, such as endocrinologists and cardiologists. Since >60% of patients with CKD are seen in a primary care setting, the role of PCPs in improving CKD care is crucial (16), engaging in diagnosis and management as early as possible to slow progression and prevent cardiovascular and kidney complications (17).
The aim of this study was to evaluate frequency of guideline-concordant CKD testing among at-risk patients. Retrospective data from a national clinical laboratory provided real-world evidence (RWE) of testing by clinicians across the U.S. and identified state-level testing variations that could inform need for education and awareness initiatives.
Research Design and Methods
Study Design
Patients >18 years of age who had testing at a Laboratory Corporation of America Holdings (Labcorp) facility between January 2013 and December 2019 were defined as at risk for CKD if they had orders containing ICD-9 or ICD-10, Clinical Modification, codes for type 1 or type 2 diabetes (250 and E10–E11, respectively) or hypertension (401 and I10, I11, and I15, respectively). To avoid CKD-diagnosed patients in the at-risk group, patients were excluded for a given and subsequent years if a CKD-associated ICD (585 or N18, respectively) was used on any order. Results were drawn from individual tests and panels, including basic and comprehensive metabolic panels or the Kidney Profile (uACR and eGFR), created in 2018 (18). Serum creatinine was reported to two decimal places to calculate eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation. The laboratory system uniformly used internationally standardized assays for serum creatinine. No race coefficient was used for eGFR calculation, as race/ethnicity data were not uniformly available.
Guideline-concordant CKD assessment was defined as the presence of both eGFR and uACR results within a given year. Urine protein-to-creatinine ratios (uPCRs) were also included; although albuminuria is preferred as the most sensitive test, proteinuria is an acceptable alternative, particularly in resource-limited settings (12–14). Urine albumin assays are currently being standardized internationally, whereas urine protein quantitative testing incorporates three biomarker assays, making standardization challenging (14). Urine protein dipstick testing from urinalysis was not included because of insensitivity and high variability based on hydration status. Testing rate was defined as the percentage of those with both tests performed out of all considered at risk within a given year, measured nationwide and statewide (states with <200 at-risk patients/year were excluded). Six-year state trends assessed with the Mann-Kendall trend test report trend direction. Median and interquartile range (IQR) better represented distributions. Regional variability of mean testing rate categorized states as Northeast, Midwest, South, or West according to U.S. Census Bureau–designated regions, compared by Kruskal-Wallis test. Significance of all tests is reported at P < 0.05.
As patients change health care professionals or locations, their tests may be routed to different laboratories. To minimize bias for these missing data and assess active Labcorp patients only, we censored patients from a given year N unless they had at least one test completed with Labcorp in years N and N+1. This was designed to exclude patients who changed ordering clinicians or laboratory. Consequently, the study reflects data from 2013 to 2018, since 2019 met the requirement for the final N+1 year.
Statistical Analysis
Data cleaning, statistical analysis, and visualization was performed using Python 3.6, SciPy 1.6.0, and Plotly 4.14. Continuous variables are presented as mean and SD or median and IQR depending on their distribution. Categorical variables are presented as percentages.
Ethical Approval
This study was approved with waiver of informed consent by the Western Institutional Review Board (number 1178401; Olympia, WA).
Results
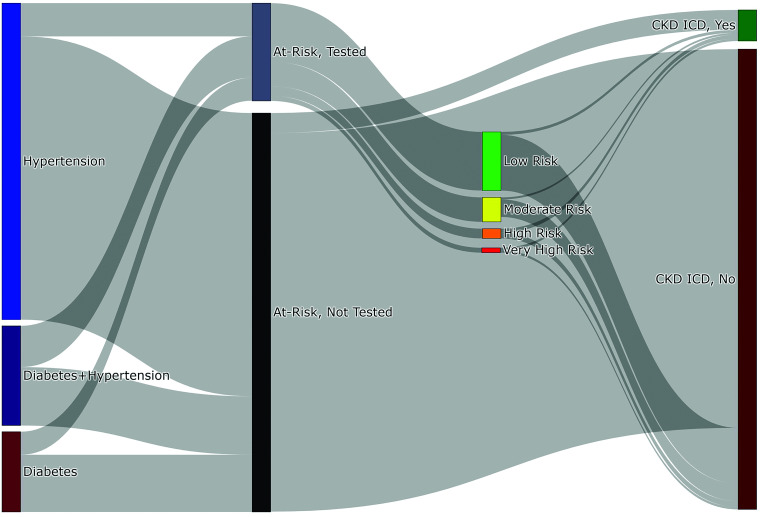
After patient censoring, 28,277,893 patients were identified as at risk for CKD during the study period. Risk conditions included 63.8% (n = 18,030,710) with hypertension only, 16.2% (n = 4,567,453) with diabetes only, and 20.1% (n = 5,679,730) with both conditions (Table 1). Complete CKD testing was identified in 19.7% of all patients (n = 5,559,434) at least once in the study period and by risk factor in 28.7% of patients with diabetes (n = 739,927), 10.5% of those with hypertension (n = 1,893,225), and 41.4% of patients with both comorbidities (n = 2,351,408). Only 21.0% of all patients had at least one uACR test (2.4% had a uPCR), whereas 89.6% of patients had an eGFR. Many with testing-confirmed CKD did not have an eventual CKD ICD (Fig. 1): 4.8% of low-risk, 10.1% of moderate-risk, 42.3% of high-risk, and 55.5% of very-high-risk individuals by KDIGO heat map classification (12).
Table 1.
Summary of patient characteristics and CKD testing
| All | Hypertension | Diabetes | Both | |
|---|---|---|---|---|
| N | 28,277,893 | 18,030,710 | 4,567,453 | 5,679,730 |
| Age (mean ± SD) at index of patient history* | 60.6 ± 14.8 | 60.7 ± 15.2 | 58.2 ± 15.1 | 62.3 ± 12.9 |
| Female (%) | 53.6 | 54.2 | 52.9 | 52.2 |
| Percentage of patients who had eGFR ordered at least once | 89.6 | 90.0 | 83.6 | 93.3 |
| eGFR (mL/min/1.73 m2), median (IQR) at index of patient history* | 89.0 (32.0) | 89.0 (31.0) | 95.0 (33.0) | 88.0 (34.0) |
| Percentage of patients who had uACR/uPCR ordered at least once | 21.0 | 11.3 | 32.2 | 43.0 |
| uACR (mg/g), median (IQR) at index of patient history* | 11.9 (32.4) | 9.6 (23.0) | 11.9 (31.5) | 14.1 (40.9) |
| Percentage of patients completely assessed (both tests) at least once | 19.7 | 10.5 | 28.7 | 41.4 |
| Cases per 6 patient-years | 1.15 | 0.58 | 1.69 | 1.98 |
Patient demographics and guideline-recommended CKD testing rates in at-risk individuals with hypertension, diabetes, or both between 2013 and 2018.
Index represents the first time a patient appears during the study time frame.
Figure 1.
Flow of diagnostic testing for patients at risk for CKD. Sankey diagram showing the CKD testing and patient history flow in at-risk individuals with hypertension, diabetes, or both throughout the study period. At-Risk, Tested is defined by the presence of eGFR and uACR in a given year during the study time frame.
The percentage of eligible patients, considering either comorbidity, completely assessed in a given state per year is shown in Table 2 (total patient count by state per year as denominators can be found in Supplementary Table 1). Hawaii, North Dakota, and Vermont did not meet the minimum 200 patient requirement and were excluded. Mann-Kendall trend tests identified increasing rates in 19 states (P < 0.05), stable rates in the remaining 28 states (P > 0.05), and no decreasing trends over the 6-year period. Outliers from Wisconsin in 2013 and 2014 reflect a reduced sample size in the state compared with later years, indicative of testing facility availability in the area. Nationwide mean testing rate increased each year from 10.7% in 2013 to 15.2% in 2018, with a 6-year mean of 13.1%. Geographical variability analysis among U.S. regions showed median testing rates (IQR) for Northeast, Midwest, South, and West regions at 13.4% (0.7%), 12.2% (7.1%), 10.5% (7.1%), and 14.0% (2.8%), respectively (Supplementary Fig. 1). Kruskal-Wallis testing identified no significant difference among the four regions (P = 0.398).
Table 2.
Testing rates of patients at risk for CKD across the U.S. from 2013 to 2018
| State | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | Mean | Trend | P |
|---|---|---|---|---|---|---|---|---|---|
| Wisconsin | 42.7 | 49.8 | 21.8 | 16.5 | 26.9 | 23.3 | 30.2 | No trend | 0.462 |
| Washington | 15.6 | 17.6 | 22.7 | 29.0 | 28.5 | 27.1 | 23.4 | No trend | 0.462 |
| Florida | 20.0 | 20.0 | 21.3 | 23.2 | 24.6 | 26.9 | 22.6 | Increasing | 0.027 |
| California | 19.5 | 19.0 | 21.4 | 23.2 | 23.9 | 24.8 | 22.0 | No trend | 0.086 |
| Texas | 16.0 | 18.2 | 20.7 | 23.1 | 24.9 | 26.2 | 21.5 | Increasing | 0.027 |
| Illinois | 14.4 | 14.8 | 17.6 | 19.8 | 21.5 | 23.8 | 18.6 | Increasing | 0.027 |
| Pennsylvania | 14.2 | 13.2 | 18.1 | 20.2 | 20.4 | 20.8 | 17.8 | No trend | 0.086 |
| Idaho | 9.8 | 12.4 | 15.6 | 20.3 | 20.1 | 19.1 | 16.2 | No trend | 0.462 |
| Maryland | 13.6 | 13.7 | 15.9 | 17.2 | 17.5 | 18.1 | 16.0 | Increasing | 0.027 |
| Missouri | 14.8 | 14.8 | 15.1 | 16.0 | 16.3 | 16.1 | 15.5 | No trend | 0.086 |
| Oregon | 10.2 | 10.6 | 13.5 | 18.1 | 18.6 | 21.4 | 15.4 | Increasing | 0.027 |
| Virginia | 12.8 | 13.5 | 14.5 | 16.4 | 17.5 | 17.7 | 15.4 | Increasing | 0.027 |
| Delaware | 14.2 | 13.7 | 15.5 | 16.4 | 16.6 | 15.7 | 15.3 | No trend | 0.462 |
| New Mexico | 11.4 | 13.0 | 18.9 | 16.7 | 14.8 | 16.5 | 15.2 | No trend | 0.221 |
| Alaska | 17.3 | 15.2 | 16.0 | 12.9 | 11.6 | 11.8 | 14.1 | No trend | 0.086 |
| Kansas | 13.4 | 12.3 | 13.2 | 13.1 | 14.7 | 16.4 | 13.8 | No trend | 0.221 |
| Colorado | 11.7 | 11.6 | 13.0 | 14.8 | 15.3 | 16.2 | 13.8 | No trend | 0.086 |
| South Dakota | 11.1 | 15.2 | 14.3 | 18.2 | 10.1 | 13.7 | 13.8 | No trend | 1.000 |
| New Hampshire | 12.0 | 11.4 | 12.6 | 14.7 | 15.9 | 15.3 | 13.7 | No trend | 0.221 |
| New Jersey | 12.0 | 12.2 | 13.3 | 14.2 | 14.7 | 15.4 | 13.6 | Increasing | 0.027 |
| Maine | 7.6 | 9.1 | 8.2 | 16.8 | 20.0 | 19.6 | 13.6 | No trend | 0.086 |
| Georgia | 10.9 | 11.5 | 13.2 | 14.0 | 14.8 | 15.1 | 13.2 | Increasing | 0.027 |
| Rhode Island | 8.7 | 9.7 | 15.0 | 16.3 | 14.6 | 14.9 | 13.2 | No trend | 0.221 |
| New York | 11.3 | 11.2 | 13.0 | 13.7 | 13.8 | 14.7 | 13.0 | No trend | 0.086 |
| Arizona | 7.4 | 8.6 | 10.8 | 13.6 | 17.7 | 19.5 | 12.9 | Increasing | 0.027 |
| Massachusetts | 11.9 | 12.0 | 13.2 | 12.4 | 12.1 | 15.9 | 12.9 | No trend | 0.086 |
| Utah | 10.5 | 11.2 | 13.0 | 13.5 | 14.8 | 14.5 | 12.9 | No trend | 0.086 |
| Nevada | 9.3 | 10.6 | 12.7 | 13.7 | 13.9 | 15.6 | 12.6 | Increasing | 0.027 |
| Ohio | 9.5 | 9.6 | 12.0 | 13.7 | 13.7 | 14.9 | 12.2 | Increasing | 0.027 |
| North Carolina | 9.0 | 9.1 | 11.3 | 13.5 | 14.1 | 14.4 | 11.9 | Increasing | 0.027 |
| Connecticut | 6.7 | 8.4 | 11.8 | 12.2 | 13.4 | 14.2 | 11.1 | Increasing | 0.027 |
| Tennessee | 9.4 | 9.2 | 10.3 | 11.0 | 11.7 | 11.9 | 10.6 | No trend | 0.086 |
| South Carolina | 7.9 | 8.6 | 9.6 | 11.4 | 12.1 | 12.8 | 10.4 | Increasing | 0.027 |
| Montana | 8.4 | 12.4 | 10.3 | 11.0 | 12.1 | 8.3 | 10.4 | No trend | 0.806 |
| West Virginia | 7.8 | 8.3 | 9.6 | 11.1 | 11.2 | 11.7 | 10.0 | Increasing | 0.027 |
| Louisiana | 8.4 | 8.0 | 9.3 | 10.3 | 11.1 | 11.3 | 9.7 | No trend | 0.086 |
| Indiana | 5.6 | 6.8 | 8.4 | 10.5 | 11.1 | 11.3 | 8.9 | Increasing | 0.027 |
| Alabama | 7.0 | 6.8 | 7.4 | 8.3 | 9.4 | 10.6 | 8.3 | No trend | 0.086 |
| Kentucky | 6.8 | 6.9 | 7.7 | 8.7 | 9.2 | 9.9 | 8.2 | Increasing | 0.027 |
| Iowa | 3.0 | 6.2 | 7.6 | 9.2 | 11.5 | 10.4 | 8.0 | No trend | 0.086 |
| Nebraska | 3.8 | 3.4 | 5.1 | 5.8 | 11.2 | 14.0 | 7.2 | No trend | 0.086 |
| Minnesota | 3.3 | 4.4 | 7.5 | 9.2 | 10.4 | 5.5 | 6.7 | No trend | 0.221 |
| Arkansas | 4.7 | 5.3 | 6.1 | 6.7 | 7.9 | 8.0 | 6.5 | Increasing | 0.027 |
| Michigan | 3.5 | 4.0 | 4.2 | 5.6 | 9.6 | 10.8 | 6.3 | Increasing | 0.027 |
| Oklahoma | 2.8 | 3.7 | 5.1 | 7.2 | 8.6 | 8.2 | 5.9 | No trend | 0.086 |
| Mississippi | 4.6 | 4.3 | 5.1 | 5.4 | 5.3 | 6.3 | 5.2 | No trend | 0.221 |
| Wyoming | 7.2 | 3.3 | 4.7 | 3.4 | 4.6 | 4.9 | 4.7 | No trend | 0.806 |
| Mean | 10.7 | 11.4 | 12.5 | 13.9 | 14.8 | 15.2 | 13.1 | Increasing | 0.027 |
Data are percentages. Table is ordered by descending mean of state testing rate over the 6-year period; nationwide rates are reported in the last row (Mean). Trends were analyzed with the Mann-Kendall trend test, with significance reported at P < 0.05.
A total of 275,335,683 eGFR tests were ordered across the cohort over 6 years versus 17,289,690 uACR and 2,018,301 uPCR orders. Of eGFR results, 98.4% came from basic or comprehensive metabolic panels and only 0.01% from the Kidney Profile. Less than 0.1% of eGFRs were direct orders compared with 98.6% of total uACR tests. The majority of tests were ordered by primary care specialties: 39.7% and 25.8% of eGFR tests by family practice (FP) and internal medicine (IM), respectively, versus 2.2% from nephrology. Similarly, 41.5% and 27.0% of uACR tests were ordered by FP and IM, respectively, versus 2.5% from nephrology. Alternatively, nephrology accounts for 54.3% of uPCR orders versus 10.0% from FP and 14.8% from IM.
Conclusions
CKD Testing Among At-Risk Adults in the U.S. Is Low
To our knowledge, this is the largest retrospective analysis of CKD testing rates, assessing >28 million at-risk patients in the U.S. Results show testing for CKD in individuals with hypertension and/or diabetes is low in routine clinical care, despite guideline recommendations. Overall testing rate of at-risk patients was <20%, which compares unfavorably to diseases like colorectal cancer, which registers a 68.8% colonoscopy rate nationwide in at-risk individuals, despite being more invasive and costly (19). Stratified by comorbidity, patients with only hypertension had the lowest rates (10.5%) of complete testing (uACR and eGFR), while patients with both conditions had the highest (41.4%). Lack of complete testing was driven primarily by absence of albuminuria testing (79.0%), rather than missing eGFR testing (10.4%).
High eGFR testing rates are confirmed from metabolic panels (>98%), which the authors speculate are typically ordered for routine-care appointments and thereby considered passive CKD testing. However, detection and risk stratification also require identification of albuminuria (12–14) and therefore would warrant an intention of the clinician to test for CKD with uACR. These findings of low albuminuria testing are similar to other published studies, but on a larger scale (20). Rates compare similarly to studies performed by other diagnostic laboratories, such as a joint study in Arizona between the National Kidney Foundation (NKF) and Sonora Quest Laboratories, which found that 79% of patients with laboratory evidence of diabetes did not have a uACR performed in the 12 months preceding the study (21). The 2019 U.S. Renal Data System (USRDS) Annual Report also identified that in 2017, only 12.9% of Medicare patients aged ≥65 years without diagnosed CKD received albumin testing; by comorbidity, 42.2% of those with diabetes alone were tested, 6.8% of those with only hypertension, and 43.2% of those with both (6). Similarly, the 2018 USRDS Annual report using data from 2016 by Optum Clinformatics commercial health insurance patients, aged 22 to 64 years, show uACR testing in 49.0% of patients with diabetes, 7.1% of patients with hypertension, and 50.7% of those with both (22). Furthermore, trial evidence supports changes in albuminuria and eGFR may serve as end points for clinical trials for those with or without diabetes or hypertension (23,24). The need for both tests and the dichotomy of order intentions highlights the usefulness of a dedicated testing panel like the Kidney Profile, which these results identify as still underutilized.
This study adds important information about state-level variability in testing rates across the U.S., ranging from <5% of at-risk patients to >30%. While Northeast and West regions exhibit higher median testing rates and smaller spreads, rates were not statistically different among the four U.S. regions, and no individual state experienced a downward trend in testing. In fact, almost half of states and the national average were found to have statistically significant increasing trends. Interestingly, compared with the Behavioral Risk Factor Surveillance System survey-level awareness study by Dharmarajan et al. (9), of the 10 states with highest awareness scores, 7 exhibited increasing trends (Arkansas, Arizona, Florida, Georgia, Michigan, Texas, and West Virginia), and in the 10 states with highest imputed CKD prevalence rates, 6 exhibited no trend in testing (Alabama, Delaware, Louisiana, Missouri, Mississippi, and Oklahoma).
Primary Care Professionals Play a Pivotal Role in CKD Care
PCPs represent the front line of early identification and management of CKD, but may not consistently identify its underlying presence (16). In a study of 5,036 patients with type 2 diabetes with CKD, only 12.1% were identified with CKD via testing by the PCP prior to study participation (25). However, as CKD stage progressed, the proportion of patients identified increased by severity (1.1% of stage G1, 4.9% for G2, 18.0% for G3, 52.9% for G4, and 58.8% for G5) and detection of CKD by the PCP was strongly associated with patient awareness of the condition.
The effectiveness of two simple, inexpensive tests for diagnosis that can be ordered by primary care offers benefits to population health and a contrast to other diseases, which may require more expensive and invasive tests for detection and risk stratification. A population health model with emphasis on CKD testing in patients with diabetes resulted in a 54% reduction in incident ESKD attributed to diabetes in a population enriched for CKD (26). Furthermore, a 2013 cost analysis study concluded that CKD medical costs are substantial among Medicare beneficiaries, increasing with severity, suggesting early identification to prevent high costs (27). Although the Kidney Profile was introduced in 2018 to streamline and facilitate CKD testing, adoption by ordering clinicians has been limited thus far.
PCP importance is demonstrated in this study, in which FP and IM providers ordered the majority of tests. Low rates of eGFR and uACR orders from nephrology could indicate that these patients have not yet been referred; high rates of uPCR, a test preferred by nephrologists, may indicate those already monitored for CKD. The main issue is the disparity in orders from PCPs: only 21.0% of patients for uACR versus 89.6% for eGFR. Previous studies have shown that low eGFR, rather than albuminuria, is the leading reason for nephrology referral (28), suggesting there may be an underappreciation of the presence of albuminuria or proteinuria and its effect on CKD progression. Barriers to proper CKD care and management could be contributing factors to these disparities: a survey of PCPs identified not only low patient awareness as a barrier, but also difficulty of maintaining current CKD guidelines and limited time and resources as main concerns, expressing a desire for better laboratory decision support and more concise guidelines as mechanisms for better care (29). Our study identifies opportunities to raise awareness of the need for testing not just in primary care, but all specialties that monitor at-risk patients, while suggesting laboratory solutions like the Kidney Profile.
Early Diagnosis and Intervention Can Improve CKD Management and Complications
Both diagnosed and undiagnosed CKD can be complicated by cardiovascular disease, kidney failure, and mortality. Effective interventions include lifestyle changes, dietary modifications, and medical management. Smoking cessation can reduce albuminuria (30). For those with diabetes, increased physical activity improves glycemic control (31) and therapeutic interventions include blood pressure and glucose control.
Benefits of ACE inhibitors or angiotensin receptor blockers (ARBs) on reducing cardiovascular risk and slowing CKD progression in hypertension with albuminuria are well established (32). This may reflect lower uACR testing in this study, as laboratory data cannot confirm existing ACE or ARB prescriptions. The American College of Physicians recommends against proteinuria testing in those with diabetes in this case, albeit graded as a weak recommendation with low-quality evidence (33). However, a study evaluating Healthcare Effectiveness Data and Information Set measures for diabetic nephropathy found only 1% of examined patients with diabetes and prescribed ACE/ARBs had albuminuria in years prior, suggesting inclusion of these medications may lead to undertesting of an at-risk population (34). Additionally, some studies indicate higher levels of uACR in those with hypertension is associated with higher likelihood of ACE inhibitor or ARB initiation, suggesting albuminuria testing can lead to better medical management (35). While eGFR assesses kidney function, uACR assesses kidney damage, and both are needed to stage CKD, underscoring their value in management.
In 2019, the U.S. Food and Drug Administration approved the first novel treatment for type 2 diabetic kidney disease in nearly 18 years: canagliflozin, a sodium–glucose cotransporter 2 inhibitor, for which effectiveness was exhibited in the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial, specifically to reduce risk of ESKD in those with uACR >300 mg/g (36). Other drugs in this class, such as dapagliflozin, have also been shown to reduce eGFR decline, albuminuria, cardiovascular events, and mortality in randomized clinical trials (37). Based on this study, in April 2021, the U.S. Food and Drug Administration also approved dapagliflozin to reduce the risk of kidney function decline, cardiovascular death, and heart failure hospitalization in those with CKD at risk for disease progression, with or without diabetes (38). Lastly, glucagon-like peptide 1 receptor agonists have shown similar improvements in cardiovascular and kidney outcomes in type 2 diabetes (39).
Effective public policies to promote early CKD diagnosis with informed interventions are critical to slow progression and reduce morbidity and mortality, while driving treatment research. Moreover, CKD has emerged as one of the most prevalent risk factors for severe COVID-19 infection, including hospitalization and mortality (40), with the CDC listing all stages as high risk.
Initiatives To Increase Awareness and Promote Guideline Adherence Are Under Way
The findings in this study highlight the need for population-based approaches to improve CKD awareness, many of which are under way. Furthermore, there has been a lack of improvement in quality of care in CKD, often not concordant with clinical practice guidelines, that emphasizes the need for new quality measures (41). Per the CDC’s reported prevalence of diabetes and hypertension, NKF is promoting the “Are you the 33%?” awareness campaign as part of the Advancing American Kidney Health Initiative in partnership with the U.S. Department of Health and Human Services and the American Society of Nephrology. In 2020, the National Committee for Quality Assurance introduced the kidney health evaluation for patients with diabetes to advance guideline-concordant testing in the U.S. as described in this study. To drive incentives for testing, NKF, in collaboration with the National Committee for Quality Assurance, developed the new 2020 Healthcare Effectiveness Data and Information Set measure for Kidney Health in Adults with Diabetes. Ongoing laboratory collaborations harmonize nationwide methodology and reporting to facilitate testing and interpretation, promoting the initiative’s goal of reducing ESKD incidence by 25% in 2030 (42). Findings in this study also help support key objectives for U.S. Department of Health and Human Services’ Healthy People 2030 in both increasing CKD awareness and decreasing prevalence. Advancing public knowledge of the current state of CKD management may help improve both patient care and research funding, which could produce novel treatments for the disease.
Strengths and Limitations
The use of RWE through clinical laboratory data reflected a variety of outpatient practice specialties, characterizing realities of testing routines in clinical care throughout the U.S. The large sample size represented a national distribution of patients and enabled state-level analysis for geographic comparisons. Longitudinal data evaluated changes in recommended testing rates over time, which improved modestly. To our knowledge, there is no other national database of this size describing at-risk patients with associated testing. While the USRDS and CDC collect data for surveillance, laboratory data are diagnostic and immediately actionable. This study represents an important novel step in analyzing large samples of CKD stratified by state to raise awareness in both patients and professionals.
Limitations of the study include identification of at-risk groups using only ICD codes accompanying laboratory orders, since neither claims data nor clinical information were available. While this makes sensitivity and specificity of diagnoses unattainable, diagnoses are likely to be specific based on existing RWE literature (43,44), especially for chronic diseases. As these ICDs likely do not reflect every diagnosis per patient, some at-risk patients were almost certainly not included, reducing sensitivity. Also, since results include only tests at Labcorp facilities, it is possible patients had testing performed with other laboratories or at point-of-care and therefore not accounted for in this study. The patient censoring model minimized this limitation and captured patients consistently tested by Labcorp. Regional differences in testing could be impacted by unknown correlations among clinician testing practices, laboratory choices, or limited market availability of Labcorp. However, given 9 of the 20 states with highest patient counts have mean testing rates below the national average, the authors speculate that regional Labcorp dominance does not govern testing rates.
Given that CKD severity was based on non-African American eGFR calculations, this may overestimate prevalence of low eGFR in a subpopulation. However, race coefficients for eGFR are controversial, with some advocating eGFR for all races as presented in this study (45). The NKF and American Society of Nephrology have convened a task force to reassess use of race in diagnosing kidney diseases that will outline a national solution and suggest alternative approaches to eGFR estimation (46). Additionally, while lack of sociodemographic data limits advanced stratification of the population, it does not obviate important findings of low CKD testing rates, as those calculated in this study depend on presence of a completed eGFR order rather than a test result that would depend on a race equation.
This large retrospective analysis provides RWE that rates of guideline-concordant CKD testing in at-risk patients remains low and has improved only modestly between 2013 and 2018. As the at-risk population for CKD grows with increasing prevalence of diabetes, hypertension, and obesity and risk for severe coronavirus disease 2019 infection rises, it is imperative to identify and treat early. These results highlight the need for improved physician detection of CKD and intervention strategies and support the utility of dedicated panels to simplify the testing process for PCPs and other clinicians who manage hypertension and diabetes.
Article Information
Acknowledgments. The authors thank Mimi Barringer (Labcorp) and Ashby Andrews and Lesley Hunter (NKF) for support in writing and coordinating efforts for this study.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.A. queried data, identified cohorts, and performed patient censoring. D.A. wrote the initial draft, and all coauthors reviewed the manuscript and agreed to publication. D.A. and S.L. performed statistical analysis. J.E., B.G., and J.A.V. provided clinical expertise. J.E., B.G., J.A.V., and S.L. supervised the study. M.J.L., E.M., and S.F. provided study criteria and knowledge of primary care and CKD guidelines. D.A. and S.L. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract and virtual poster form at the National Kidney Foundation 2020 Spring Clinical Meeting, 25–29 March 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14748867.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1. Murphy D, McCulloch CE, Lin F, et al.; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team . Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 2016;165:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Kidney Foundation . 2020 Chronic Kidney Disease Fact Sheet. Accessed 26 February 2021. Available from https://www.nkfm.org/sites/default/files/documents/pages/ckd_fact_sheet_-_ nkfm_feb_2020.pdf
- 3. Hoerger TJ, Simpson SA, Yarnoff BO, et al. The future burden of CKD in the United States: a simulation model for the CDC CKD Initiative. Am J Kidney Dis 2015;65:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghaderian SB, Hayati F, Shayanpour S, Beladi Mousavi SS. Diabetes and end-stage renal disease; a review article on new concepts. J Renal Inj Prev 2015;4:28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vassalotti JA, DeVinney R, Lukasik S, et al. CKD quality improvement intervention with PCMH integration: health plan results. Am J Manag Care 2019;25:e326–e333 [PubMed] [Google Scholar]
- 6. United States Renal Data System . 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Accessed 26 February 2021. Available from https://www.usrds.org/media/2371/2019-executive-summary.pdf
- 7. Chu CD, McCulloch CE, Banerjee T, et al.; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team . CKD awareness among US adults by future risk of kidney failure. Am J Kidney Dis 2020;76:174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Plantinga LC, Crews DC, Coresh J, et al.; CDC CKD Surveillance Team . Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol 2010;5:673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dharmarajan SH, Bragg-Gresham JL, Morgenstern H, et al.; Centers for Disease Control and Prevention CKD Surveillance System . State-level awareness of chronic kidney disease in the U.S. Am J Prev Med 2017;53:300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention . National Chronic Kidney Disease Fact Sheet 2017. U.S. Department of Health and Human Services. Accessed 26 February 2021. Available from https://www.cdc.gov/diabetes/pubs/pdf/kidney_fact sheet.pdf
- 11. Molnar AO, Hiremath S, Brown PA, Akbari A. Risk factors for unplanned and crash dialysis starts: a protocol for a systematic review and meta-analysis. Syst Rev 2016;5:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013;3:1–150 [Google Scholar]
- 13. American Diabetes Association . 10. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S105–S118 [DOI] [PubMed] [Google Scholar]
- 14. Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713–735 [DOI] [PubMed] [Google Scholar]
- 15. Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006;17:2974–2984 [DOI] [PubMed] [Google Scholar]
- 16. Allen AS, Forman JP, Orav EJ, Bates DW, Denker BM, Sequist TD. Primary care management of chronic kidney disease. J Gen Intern Med 2011;26:386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vassalotti JA, Fox CH, Becker BN. Risk factors and screening for chronic kidney disease. Adv Chronic Kidney Dis 2010;17:237–245 [DOI] [PubMed] [Google Scholar]
- 18. Miller WG, Bachmann LM, Delanghe JR, Inker LA, Jones GRD, Vassalotti JA. Optimal use of biomarkers for chronic kidney disease. Clin Chem 2019;65:949–955 [DOI] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention . Behavioral Risk Factor Surveillance System Survey Data. Atlanta, GA, Centers for Disease Control and Prevention, 2018 [Google Scholar]
- 20. Hallan SI, Dahl K, Oien CM, et al. Screening strategies for chronic kidney disease in the general population: follow-up of cross sectional health survey. BMJ 2006;333:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Montgomery E, Clark J, Ivie J, Zeigler K, Cohen R. Laboratory Medicine Impact on Population Health Implementing the Kidney Profile. National Kidney Foundation, 2019. Accessed 28 May 2021. Available from https://www.kidney.org/sites/default/files/02-11-8160_ hbj_cmo_ascp_poster3.pdf
- 22. United States Renal Data System . 2018 USRDS Annual Data Report: Executive Summary. Am J Kidney Dis 2019;73(Suppl. 1):A9–A22 [Google Scholar]
- 23. Gerstein HC, Mann JFE, Yi Q, et al.; HOPE Study Investigators . Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001;286:421–426 [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 2020;75:84–104 [DOI] [PubMed] [Google Scholar]
- 25. Szczech LA, Stewart RC, Su HL, et al. Primary care detection of chronic kidney disease in adults with type-2 diabetes: the ADD-CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease). PLoS One 2014;9:e110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Narva A. Population health for CKD and diabetes: lessons from the Indian Health Service. Am J Kidney Dis 2018;71:407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol 2013;24:1478–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McIntyre NJ, Fluck R, McIntyre C, Taal M. Treatment needs and diagnosis awareness in primary care patients with chronic kidney disease. Br J Gen Pract 2012;62:e227–e232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sperati CJ, Soman S, Agrawal V, et al.; National Kidney Foundation Education Committee . Primary care physicians’ perceptions of barriers and facilitators to management of chronic kidney disease: A mixed methods study. PLoS One 2019;14:e0221325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roehm B, Simoni J, Pruszynski J, Wesson DE. Cigarette smoking attenuates kidney protection by angiotensin-converting enzyme inhibition in nondiabetic chronic kidney disease. Am J Nephrol 2017;46:260–267 [DOI] [PubMed] [Google Scholar]
- 31. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011;26:2827–2847 [DOI] [PubMed] [Google Scholar]
- 33. Qaseem A, Hopkins RH Jr, Sweet DE, Starkey M; Clinical Guidelines Committee of the American College of Physicians . Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2013;159:835–847 [DOI] [PubMed] [Google Scholar]
- 34. Krause TM, Ganduglia-Cazaban C, Finkel KW. Rates for HEDIS screening for diabetic nephropathy quality measure may be overstated. Manag Care 2018;27:45–49 [PubMed] [Google Scholar]
- 35. Qiao Y, Shin JI, Chen TK, et al. Association of albuminuria levels with the prescription of renin-angiotensin system blockade. Hypertension 2020;76:1762–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 37. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 38. U.S. Food and Drug Administration . FDA Approves Treatment for Chronic Kidney Disease. Accessed 28 May 2021. Available from https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-chronic-kidney-disease
- 39. American Diabetes Association . 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S111–S134 [DOI] [PubMed] [Google Scholar]
- 40. ERA-EDTA Council; ERACODA Working Group . Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant 2021;36:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tummalapalli SL, Powe NR, Keyhani S. Trends in quality of care for patients with CKD in the United States. Clin J Am Soc Nephrol 2019;14:1142–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. U.S. Department of Health and Human Services . Advancing American Kidney Health. Accessed 26 February 2021. Available from https://aspe.hhs.gov/system/files/pdf/262046/Advancing AmericanKidneyHealth.pdf
- 43. O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res 2005;40:1620–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ravizza S, Huschto T, Adamov A, et al. Predicting the early risk of chronic kidney disease in patients with diabetes using real-world data. Nat Med 2019;25:57–59 [DOI] [PubMed] [Google Scholar]
- 45. Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA 2019;322:113–114 [DOI] [PubMed] [Google Scholar]
- 46. Delgado C, Baweja M, Burrows NR, et al. Reassessing the inclusion of race in diagnosing kidney diseases: an interim report from the NKF-ASN Task Force. J Am Soc Nephrol 2021;32: 1305–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]