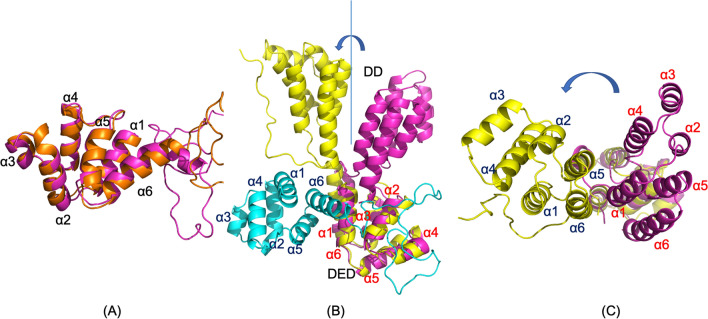
Figure 4.
The simulated complex structure of PEA-15pp/FADD. (A) PEA-15pp in the complex (orange) is superimposed with free-form PEA-15pp (magenta) with the RMSD of 0.803 Å before the kink on helix α6. There is no significant conformational change between the free and bound forms. (B) FADD in the complex (yellow) is superimposed with the NMR structure of intact FADD, 2GF5 (magenta), over DED residues (RMSD 2.118 Å). PEA-15pp in the complex is colored cyan. DED–DED interaction in the complex is orthogonal in directions. The FADD DD in the complex rotated about 90° from the free form around the linker between the two domains. (C) same as (B) but observed from the top of FADD DD to illustrate the relative positions of the six helices on DD. PEA-15pp is not shown for clarity. The molecular structures were visualized and generated using PyMOL version 2.4 (http://www.pymol.org/).