FIGURE 1.
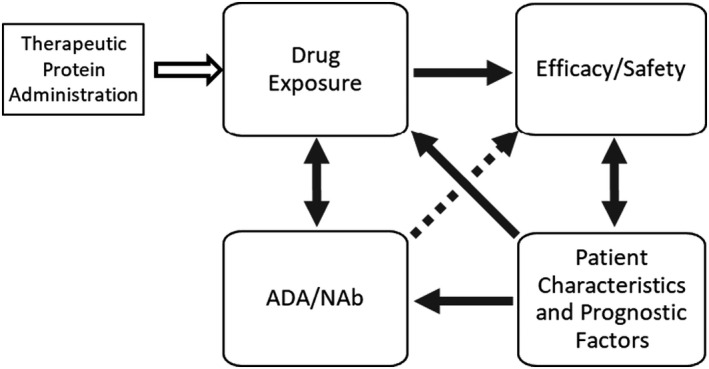
ADA assessment framework for oncology biologics. Immunogenicity assessment in oncology should consider the complex relationship of drug concentrations, ADA/NAb, efficacy, and patient characteristics and prognostic factors. The direction of the arrows indicates the direction of the impact, which can be unidirectional or bidirectional. The dotted arrow represents the hypothetical effect of ADA directly on efficacy without impacting drug exposure. ADA, anti‐drug antibody; NAb, neutralizing antibody
