Abstract
Tacrolimus is the key component of most contemporary immunosuppressive drug regimens for the prevention of transplant rejection. Area under the concentration time curve over 24 h (AUC0–24) predicts efficacy, but predose (trough) tacrolimus blood concentration (C0) is currently used to guide dosing. In clinical or research situations where an estimate of AUC is required, collection of a full 24 h pharmacokinetic (PK) profile is cumbersome. Limited sampling strategies (LSSs) have been developed for some tacrolimus preparations but not for the new, extended‐release, once‐daily formulation of tacrolimus, ENVARSUS XR. Twenty‐four kidney transplant recipients were enrolled in this study. Twenty‐four tacrolimus PK profiles were obtained over 24 h. Multiple linear regression was used to generate LSSs with the best subset selection for accurate estimation of tacrolimus AUC0–24. The predictive performance of each model was assessed in the evaluation group. The correlation between actual and predicted AUC0–24 was evaluated and mean percentage prediction error (MPE%), mean absolute percentage prediction error (MAE%), and root mean squared error (RMSE) were calculated for each prediction model to assess bias and precision. The selected LSSs were highly correlated to AUC0–24 compared with the correlation between C0 and AUC0‐24. Two and three sampling points limited sampling strategies: C0, C2, and C10 provide the most reliable and effective LSS for estimation of tacrolimus AUC0–24 in routine clinic use. These limited sampling models can be applied in therapeutic drug monitoring schemes to personalize tacrolimus dosing for kidney transplant recipients on treatment with extended‐release tacrolimus.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Tacrolimus is a narrow therapeutic index drug with wide pharmacokinetic (PK) variability between individuals. There is evidence that tacrolimus exposure based on blood concentration (C0) monitoring can vary extensively. Although AUC is considered the best exposure indicator related to tacrolimus clinical effects, tacrolimus C0 monitoring is the most commonly used method in routine clinical practice. There is no limited sampling strategy (LSS) available for kidney transplant recipients using extended‐released tacrolimus.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study assessed different LSSs for an accurate estimation of area under the concentration time curve over 24 h (AUC0–24) for extended‐released tacrolimus.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Tacrolimus exposure can be accurately measured using two and three sampling points of LSS in kidney transplant recipients using extended‐released tacrolimus.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
These LSSs can be applied in therapeutic drug monitoring (TDM) schemes to personalize tacrolimus dosing in routine clinical practice for kidney transplant recipients receiving extended‐released tacrolimus. Moreover, it can be used in PK clinical trials providing an accurate AUC0–24 estimation. It also can have useful implementation in the new micro‐sampling techniques.
INTRODUCTION
Tacrolimus is a cornerstone of the immunosuppressive drug regimens used for solid organ transplantation. Tacrolimus has a narrow therapeutic window and its high pharmacokinetic (PK) variability renders dose selection challenging. To a certain extent, it is genetically related. 1 Therefore, regular monitoring and adjustment of tacrolimus dose is crucial to prevent rejection and adverse effects associated with toxic drug concentrations. 2 Efficacy and side effects of immunosuppressive drugs are highly correlated with the area under the concentration time curve (AUC). However, recording a full PK profile for every patient is not feasible in clinical practice. 3 , 4 Consequently, predose trough concentration (C0) monitoring is the most widely used method to guide tacrolimus dosing in routine clinical practice. Despite the simplicity of this method, which provides a high correlation with AUC, some concerns have been raised about therapeutic drug monitoring (TDM) accuracy based on C0 with tacrolimus AUC reported to vary by up to twofold for the same C0 concentration. 5 Moreover, there are conflicting data relating tacrolimus C0 to clinical outcomes. Toxicity and rejection sometimes occur within the target range. 6
In clinical or research situations where a greater degree of confidence in predicting the AUC than can be provided by C0 monitoring, limited sampling strategies (LSSs) offer a practical alternative to collecting full 24 h PK profiles. LSS include a limited number of samples, such as trough and time points within a short time post‐dose. 7 Collection of full 24 h PK profiles is both costly and likely to be a deterrent to subject recruitment to clinical trials.
ENVARSUS XR is an extended‐release formulation of tacrolimus that has been licensed for routine use recently. It has been designed using MeltDose drug delivery technology to allow sustained release of the drug as it passes along the intestine, rather than the rapid absorption that occurs with immediate release twice daily tacrolimus formulations. extended‐release tacrolimus formulation has shown equivalent efficacy and safety to twice daily tacrolimus in de novo and stable kidney transplant recipients 8 , 9 and to once daily tacrolimus (ADVAGRAF) in stable kidney transplant recipients. 9 It achieves equivalent drug exposure over 24 h to once and twice‐daily tacrolimus with at least a 30% dose reduction requirement and a significant decrease in tacrolimus maximum concentration (Cmax) in the blood. 10 LSSs to estimate AUC0–24 have been developed for other tacrolimus preparations (Armendáriz, et al. 11 2005; Mathew et al. 7 2008; and van Boekel et al. 4 2015). However, limited data are available for an appropriate LSS for kidney transplant recipients who use once‐daily tacrolimus formulation, ENVARSUS XR. Therefore, the purpose of the study was to develop a clinically precise and applicable LSS to estimate the area under the curve (AUC0–24) of modified‐release tacrolimus.
PATIENTS AND METHODS
Patients
A total of 24 stable kidney transplant recipients were recruited for the study. All patients received a stable dose of twice‐daily tacrolimus (ADOPORT; Sandoz Limited) or once‐daily tacrolimus (ADVAGRAF; Astellas, Ireland) and no more than 5 mg prednisolone daily with either mycophenolate or azathioprine as part of their immunosuppressive regimen. The conversion ratio target from ADOPORT or ADVAGRAF to ENVARSUS XR was 0.7 or 0.85 for subjects of Black ethnicity (genetically sub‐Saharan African). The total daily dose was given as a single dose in the morning (8:00 a.m.) during the study period with adjustment of the dose to maintain 24 h postdose C0 concentration in the range 4–9 μg/L for at least 2 weeks prior to blood sample collection for the 24 h PK profile. Inclusion criteria required that the participants were at least 18 years old and at least 12 weeks after transplantation. Exclusion criteria included lactose intolerance and treatment with potent cytochrome P4503A and P‐glycoprotein inducers or inhibitors.
For PK sampling, full tacrolimus AUC0–24 with 13 blood samples was collected at 0 (C0), 0.5 (C0.5), 1 (C1), 1.5 (C1.5), 2 (C2), 2.5 (C2.5), 3 (C3), 4 (C4), 6 (C6), 8 (C8), 10 (C10), 12 (C12), and 24 (C24) h after morning tacrolimus dose. Five mL of blood was collected at each time point into EDTA tubes after at least 2 weeks of stable tacrolimus dose. Whole blood samples were stored at −20°C until analyzed. The study protocol was approved by National Research Ethics Service Committee (REC Number: 18/LO/0524) and all study participants provided written informed consent.
Analytical methods
Tacrolimus blood samples were measured using a validated liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) method with a lower limit of quantitation (LLOQ) of 0.25 µg/L. The calibration range was 1–50 µg/L with a correlation coefficient (r) of 0.998. Tacrolimus was extracted from 50 µL blood using liquid‐liquid extraction. Ascomycin (50 µL, ~50 µg/L) was used as internal standard. The extraction procedure was performed using 1 ml of precipitating solution (500 ml zinc sulphate solution (25 g/L); 200 mL of acetonitrile; and 300 ml methanol). After mixing and centrifugation, the supernatant was decanted into clean tubes with 100 µL of 0.1 mol/L sodium hydroxide and 2 ml of Methyl‐tert‐butyl ether. After mixing and centrifugation, the organic solvent was evaporated to dryness, reconstituted with 250 µL of 80% methanol, and 10 µL injected into the LC‐MS/MS. An API4000 triple quadruple mass spectrometer equipped with a turbo‐ion spray was used in positive mode. The transitions selected were m/z 821.5/768.5 for tacrolimus and m/z 809.5/756.5 for ascomycin. Nitrogen was used as the collision gas. Gas settings in mL/min were: collision gas: 8, curtain gas: 10, ion source gas 1: 35, and ion source gas 2: 30. Ion spray voltage: 5500 V, and temperature: 450°C. Entrance potential (EP), collision energy (CE), and collision cell exit potential (CXP) in voltage were 10, 29, and 18 V, respectively. The dwell times were set at 300 msec. Declustering potential (DP) was 81 for tacrolimus and 76 for ascomycin. Chromatographic separation was performed using Agilent HPLC and Alltima C18, 5 µm, 150 × 2.1 mm column (Merck [BDH] Limited, Poole, Dorset, England) at a temperature of 65°C. The mobile phase was pumped isocratically at a flow rate of 0.4 ml/min and consisted of a mixture of mobile phase A (methanol) and mobile phase B (de‐ionized water +5 ml/L 2 M ammonium acetate) at a ratio of 82:18, respectively. Tacrolimus and internal standard were eluted at retention times 2.9 and 2.8, respectively.
Pharmacokinetic parameters
The area under the whole blood concentration‐time curve (AUC) was calculated using the linear trapezoidal method. The peak plasma concentration (C max) and the time to achieve Cmax (T max) were obtained from direct visual inspection of plasma concentration versus time curves. Descriptive statistics for variables were reported as mean ± SD or median (range). A correlation coefficient test was used to assess the correlation between C0 and AUC0–24.
LSS development and validation
Limited sampling strategy techniques aim to predict the AUC0–24 using an equation and a small number of blood samples collected at specific times. The evaluation for each LSS method was carried out by repeated cross validation. The evaluation method included the following steps:
-
a
The data set was repeatedly and randomly divided into two subsets. One was used to fit the model between tacrolimus AUC0–24 and each of the created linear regression models. This group was called the training group (n = 15) and the other group was used to test the model; the testing or evaluation group (n = 9). This process was repeated 50 times. Each time tacrolimus concentrations at the selected sampling time points for the 15 PK profiles in the training group were used to fit to the created linear regression models to tacrolimus AUC0–24 using multiple linear regression analysis. The equations produced were as follows:
Where β1–βn are the regression coefficients, I is the intercept, n is the nominal sample collection time, and c 1–c n are tacrolimus concentrations measured at times 1‐n for each of the regression models.
-
b
Best subsets regression was applied to derive the possible limited sampling equations. The best equations were selected for each of the 1, 2, and 3 time sampling point equations within 10 h after drug administration depending on the coefficient of determination (r 2).
-
c
The linear regression models obtained from the training group were used to estimate and validate tacrolimus AUC0–24 for the remaining 9 PK profiles in the evaluation group. Additionally, a limited sampling model for C0 was used in the validation.
-
d
The predictive performances of these models were then assessed. The predicted AUC0–24 calculated using each limited sampling strategy was compared to actual AUC0–24 calculated using the linear trapezoidal method.
Statistical analysis
The Anderson‐Darling test was used to assess the normality of the data distribution. Descriptive statistics were performed using mean ± SD or median (range). Linear regression analysis was used to compare the predicted AUC0–24 from each model against the actual AUC0–24. 12 Prediction error (PE) was calculated for each patient using the equation:
The potential predictions bias was evaluated using mean percentage prediction error (MPE%). MPE% was determined using the equation:
The precision of the predictions was assessed using mean absolute percentage prediction error (MAE%) and root mean squared prediction error (RMSE) for each individual model. MAE% and RMSE was calculated using the following equations:
Statistics were carried out using 95% confidence intervals. 13 Bland–Altman plots were used to analyze the agreement between predicted AUC0–24 and actual AUC0–24. A Pearson correlation coefficient test was used to assess the correlation between predicted AUC0–24 and actual AUC0–24. The acceptable limits for the prediction model evaluation were less than or equal to 5%, less than or equal to 10%, and less than or equal to 15% for MPE%, MAE%, and RMSE, respectively. 14 , 15
Statistical analysis was performed using software package Minitab 19 (Minitab software, USA) and Excel 2010 (Microsoft).
RESULTS
Baseline characteristics
Of 100 stable kidney transplant patients who were screened, 21 were considered not eligible for participation, 40 declined to participate, and 14 did not respond to follow‐up calls. Therefore, 25 patients were recruited for the study and 24 participants completed a full PK profile. One patient was discontinued from the study due to a moderate side effect (diarrhea). Reported or noted adverse events were diarrhea, 1 serum creatinine fluctuation, 2 and high blood pressure. 1
The characteristics of the 24 patients included in the study are shown in Table 1. All transplant recipients were at least 5 months post‐transplantation. The mean age of recipients was 53 ± 12 years. Concomitant immunosuppression included prednisolone (n = 9), mycophenolate mofetil (n = 11), and azathioprine (n = 8). At baseline, 14 patients (58%) were receiving ADOPORT and 10 patients (42%) were receiving ADVAGRAF. The median daily dose of ADVAGRAF was 4.50 mg (range 1.50–10.00), whereas the median daily dose of ADOPORT was 3.50 mg (range 1.00–30.00). Regarding self‐declared ethnic origin, 15 patients were from White backgrounds, 4 Asian, 4 Black, and 1 other ethnic background.
TABLE 1.
Patients’ demographics and clinical characteristics (n = 24)
| Characteristics | Results |
|---|---|
| Sex, male/female | 18/6 |
| Age, years, mean, ± SD | 52.75 ± 11.77 |
| Weight, kg, mean, ± SD | 76.91 ± 16.95 |
| Height, m, mean, ± SD | 1.68 ± 0.08 |
| Time after transplantation, years, mean, ± SD | 7.07 ± 4.97 |
| eGFR, ml/min/1.73 m2*, mean, ± SD | 52.38 ± 19.37 |
| Serum creatinine, µmol/L, median (range) | 133.5 (65–393) |
| Serum albumin, g/L, mean, ± SD | 38.29 ± 3.42 |
| Hematocrit, mean, ± SD | 0.39 ± 0.06 |
| Diabetes mellitus, n | 5 (21%) |
| Immunosuppression at baseline | |
| Tacrolimus, n | |
| Adoport/Advagraf | 14 (58%)/10 (42%) |
| Steroids, n | 9 (38%) |
| Mycophenolate mofetil, n | 11 (46%) |
| Azathioprine, n | 8 (33%) |
| Donor type, n | |
| Living | 10 (42%) |
| Deceased | 14 (58%) |
| Ethnicity, n | |
| White | 15 (63%) |
| Black | 4 (17%) |
| Asian | 4 (17%) |
| Other | 1 (4%) |
| Original renal disease, n (%) | |
| IgA nephropathy | 5 (21%) |
| Focal segmental glomerulosclerosis | 5 (21%) |
| Glomerulonephritis | 3 (13%) |
| Hypertension | 2 (8%) |
| Diabetes | 2 (8%) |
| Granulomatosis with polyangiitis | 1 (4%) |
| Pyelonephritis | 1 (4%) |
| Polycystic kidneys | 1 (4%) |
| Congenital renal dysplasia | 1 (4%) |
| ESRD secondary to bilateral nephrectomies | 1 (4%) |
| Uncertain etiology | 1 (4%) |
Abbreviations: eGFR, estimated glomerular filtration rate; ESRD, endstage renal disease.
The eGFR was determined using Chronic Kidney Disease Epidemiology Collaboration calculation.
Pharmacokinetic parameters
PK parameters of extended‐release tacrolimus are summarized in Table 2. All patients’ tacrolimus blood concentrations were within the therapeutic window with C0 of 5–8 µg/L. C0 was checked and adjusted before carrying out tacrolimus PK profiles for patient’s safety. The median daily dose was 2.73 mg (range 0.75–25.5). The geometric mean (95% confidence interval) for Cmax, AUC0–24, and C0 were 12.32 µg/L (10.77–13.87), 192.29 µg*h/L (173.72–210.86) and 5.79 µg/L (5.10–6.49), respectively.
TABLE 2.
Summary of tacrolimus PK parameters (n = 24)
| Variable | Mean | SD | CV% | Median | Range, min–max |
|---|---|---|---|---|---|
| Cmax, µg/L | 12.32 | 3.67 | 29.76 | 11.64 | 6.95–22.07 |
| Tmax, h | 5.15 | 2.24 | 43.59 | 5.00 | 2.00–8.00 |
| AUC0–24, µg*h/L | 192.29 | 43.98 | 22.87 | 191.88 | 138.78–305.75 |
| Dose, mg | 4.53 | 5.15 | 113.69 | 2.73 | 0.75–25.50 |
| C0, µg/L | 5.79 | 1.65 | 28.41 | 5.33 | 3.10–9.49 |
| C0.5, µg/L | 5.93 | 1.36 | 22.85 | 5.76 | 4.27–8.89 |
| C1, µg/L | 6.81 | 1.94 | 28.50 | 6.51 | 4.02–10.64 |
| C1.5, µg/L | 7.51 | 2.79 | 37.08 | 6.90 | 4.06–15.49 |
| C2, µg/L | 8.27 | 3.31 | 40.01 | 7.44 | 4.11–16.23 |
| C2.5, µg/L | 9.07 | 3.81 | 42.05 | 8.03 | 4.68–22.07 |
| C3, µg/L | 9.78 | 3.61 | 36.91 | 9.03 | 4.85–18.82 |
| C4, µg/L | 10.24 | 3.66 | 35.73 | 9.51 | 5.94–18.08 |
| C6, µg/L | 10.65 | 2.97 | 27.87 | 10.73 | 6.02–16.51 |
| C8, µg/L | 9.74 | 2.54 | 26.08 | 9.37 | 6.00–15.04 |
| C10, µg/L | 8.85 | 2.29 | 25.92 | 8.53 | 5.68–15.44 |
| C12, µg/L | 8.09 | 2.07 | 25.56 | 7.71 | 5.54–13.48 |
| C24, µg/L | 5.71 | 1.40 | 24.50 | 5.32 | 3.60–9.30 |
Abbreviations: AUC0–24, area under the time‐concentration curve in a 24 h period; C0 to C24, the concentrations at 0 to 24 h; Cmax, maximum concentration; CV%, coefficient of variation; Tmax, time for reaching maximum concentration.
Limited sampling strategy equations for tacrolimus
Regression models with various sampling time points and their combinations were generated for AUC0–24 prediction using the concentration‐time data of the training set. Five predictive models consisting of one to three sampling points with the coefficient of determination (r 2) are shown in Table 3. The commonly used trough C0 to predict tacrolimus exposure (AUC0–24) provided r 2 value of 0.70. One sampling time point model at 10 h yielded an acceptable r 2 value of 0.91. Limited sampling models based on two sampling points at 0 and 10 h, and 2 and 10 h displayed r 2 values of 0.92 and 0.97, respectively. Limited sampling model using the time points at 0, 2, and 10 h provided the highest r 2 value.
TABLE 3.
Limited sampling strategies to predict tacrolimus AUC0–24 in adult kidney transplant recipients
| Model | Equation | r 2 |
|---|---|---|
| Model 1 | AUC0–24 = 64.73 + 21.62 T0 | 0.70 |
| Model 2 | AUC0–24 = 31.55 + 18.18 T10 | 0.91 |
| Model 3 | AUC0–24 = 26.38 + 5.67 T0 + 15.02 T10 | 0.92 |
| Model 4 | AUC0–24 = 21.95 + 3.90 T2 + 15.59 T10 | 0.97 |
| Model 5 | AUC0–24 = 20.26 + 3.19 T0 + 3.63 T2 + 14.01 T10 | 0.98 |
Abbreviation: AUC0–24, area under the time‐concentration curve in a 24 h period.
There were no relevant effects of hematocrit, dose, sex, ethnicity, time after transplantation, or co‐administration of steroids, on correlation of determination (r 2).
Predictive performance of limited sampling methods for tacrolimus
Table 4 summarizes the predictive performance of each of the limited sampling strategies. Bias and precision were assessed using the evaluation set (n = 9). All equations showed a better correlation with AUC0–24 than C0. The limited sampling model that incorporated C0 measurements (model 1) demonstrated the lowest correlation of all the limited sampling models (r 2 = 0.84). It resulted in an acceptable bias (MPE%, −0.12) and the highest imprecision (MAE% 12.43% and RMSE 27.59%) compared with the other LSS models. A strong correlation was found using the LSS equation included C10 (model 2) for estimation of AUC0–24 (r 2 = 0.95). MPE%, MAE5 and RMSE were within the acceptable limits. Two sampling point models using C0 and C10 measurements (model 3), and C2 and C10 measurements (model 4) were acceptable and predictive of tacrolimus AUC0–24 based on bias and precision assessment. The performance of model 5 with sampling time points at 0, 2, and 10 h displayed the least bias and the most precise estimation to all equations (r 2 = 0.99, MPE% −0.13, MAE%, 3.44, and RMSE 7.94). Figure 1 shows the Bland‐Atman plots for models 2 , 3 , 4 , 5 that illustrates the bias and precision for each model. Limits of agreement (LOA) included 95% of the differences between predicted and actual tacrolimus AUC0–24. There were no significant differences between tacrolimus measurements using these models. Moreover, there was no consistent direction of the bias pattern with predicted AUC0–24 values were above and below the actual values. Figure 2 demonstrates the correlations between predicted AUC0–24 and actual AUC0–24 for each participant using LSS models within the acceptable limits. Models 4 and 5 had high r 2 value and similar bias and precision.
TABLE 4.
Predictive performance of limited sampling strategy models for estimation of tacrolimus exposure in adult kidney transplant recipients
| Model | Sample times (h) | Mean AUCpredicted (µg*h/L), (range) | MPE (%) (95% CI) | MAE (%) (95% CI) | RMSE (95% CI) | Agreement – concordance correlation coefficient (95% CI) |
|---|---|---|---|---|---|---|
| Model 1 | 0 h | 189.96 (131.75–269.9) | −0.12 (−2.13 to 1.90) | 12.43 (11.67–13.19) | 27.59 (25.81–29.37) | 0.84 (0.65–0.93) |
| Model 2 | 10 h | 192.38 (134.81–312.25) | 0.54 (−0.31 to 1.39) | 5.66 (5.30–6.01) | 13.98 (13.18–14.78) | 0.95 (0.89–0.98) |
| Model 3 | 0, 10 h | 192.10 (136.47–312.10) | 0.13 (−0.74 to 1.00) | 5.54 (5.12–5.96) | 14.03 (13.08–14.97) | 0.96 (0.91–0.98) |
| Model 4 | 2, 10 h | 192.10 (134.06–307.78) | −0.09 (−0.69 to 0.52) | 3.82 (3.59–4.04) | 8.66 (8.15–9.17) | 0.99 (0.97–0.99) |
| Model 5 | 0, 2, 10 h | 192.68 (135.7–308.85) | −0.13 (−0.64 to 0.39) | 3.44 (3.20–3.68) | 7.94 (7.42–8.45) | 0.99 (0.97–1.00) |
Abbreviations: AUC, area under the time‐concentration curve; AUCpredicted, predicted AUC0–24; CI, confidence interval; MAE%, mean absolute percentage prediction error; MPE%, mean percentage prediction error; RMSE, root mean squared prediction error.
Evaluation set data: n = 9; actual AUC0–24 mean (SD) 192.29 (43.98) µg*h/L; range 138.78–305.76 µg*h/L.
FIGURE 1.
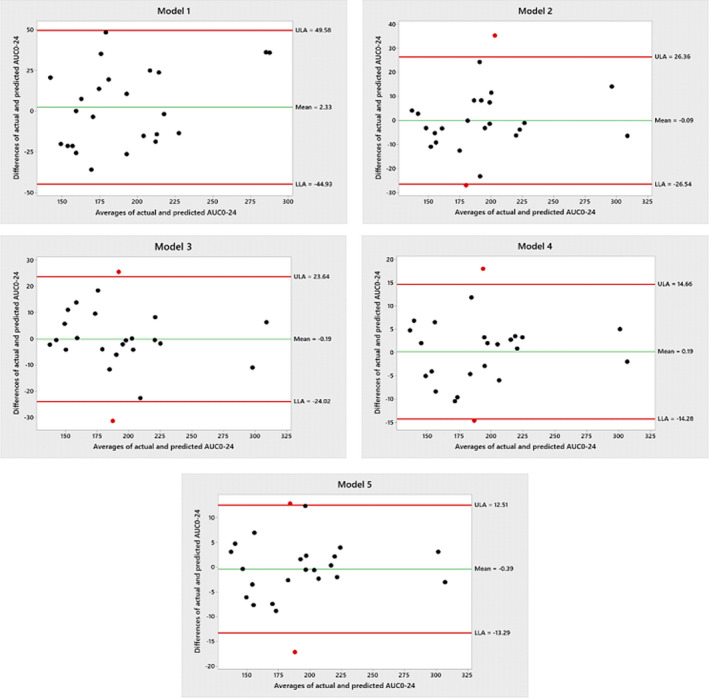
Bland‐Altman plot comparing the differences and averages of the AUCActual and AUCPredicted with the center line represents the mean difference and the red lines represent limits of agreement (LOA) with 95% confidence intervals around the mean difference. AUC0–24, area under the concentration time curve over 24 h
FIGURE 2.
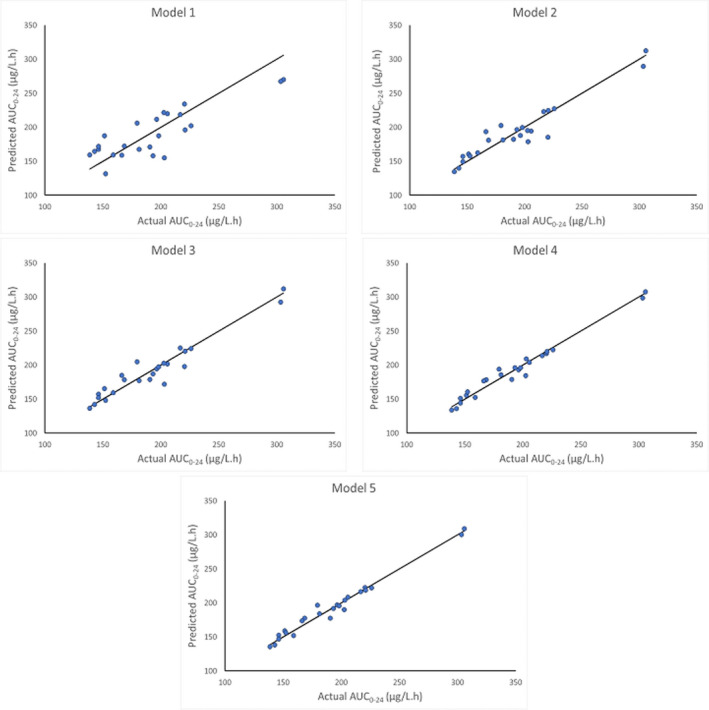
The correlation between predicted AUC0–24 and actual AUC0–24. AUC0–24, area under the concentration time curve over 24 h
DISCUSSION
To our knowledge, this study is the first to be reported developing and validating an LSS using regression equations to predict the AUC0–24 of the extended‐released tacrolimus formulation in adult kidney transplant recipients. In general, C0 is routinely used in tacrolimus dose adjustment in clinical practice. However, this study showed a moderate correlation between C0 and AUC0–24 (r 2 = 0.84) and it did not meet the precision criteria. This result revealed that the LSS model including the single time point C0 is a suboptimal marker for prediction of tacrolimus AUC0–24, although it is probably sufficiently accurate for most routine clinical situations. The prediction limited sampling formula for the AUC0–24 using the single C10 time point gave the highest correlation with the actual AUC0–24 (r 2 = 0.95). However, therapeutic drug monitoring using C10 with morning dosing would be challenging to apply in clinical practice. LSS using C0 and C10 showed a slight improvement in the model correlation (r 2 = 0.96) compared with using C10 alone. Another LSS model including C2 and C10 represented a good improvement in the model performance, including bias and precision compared with using C0 and C10.
This model provided more reliable and accurate estimation of the tacrolimus AUC0–24. When LSS model with three sample points (C0, C2, and C10) were used, the correlation between the predicted and actual AUC0–24 (r 2) was almost equal to the correlation obtained using two points LSS model (C2 and C10). Therefore, LSS with 2 time points 2 and 10 h would provide sufficient accuracy for prediction of tacrolimus AUC0–24 in clinical practice where a true measure of AUC is required or in clinical trials. The two or three sample point models would be useful to minimize the number of samples required for tacrolimus PK profile from 10 to 13 samples to 2 or 3 samples which is more convenient and cost‐effective.
This study has some limitations. This is a relatively small sample size. A further limitation is that concentration time points indicated by the models may not be accurately reflective with a relatively small shift in the collection time. In addition, this method is suitable for stable kidney transplant recipients more than 3 months post‐transplantation. More studies are required for patients with varying renal function and tacrolimus concentrations particularly in the first 3 months post‐transplant when more critical estimation of tacrolimus concentrations is required.
In conclusion, the validated LSS algorithms from this study could prove a very useful tool in therapeutic drug monitoring for prediction of the systemic exposure of extended release tacrolimus in clinical practice. Moreover, they could be used in PK clinical trials providing a practical and precise approach to estimate the AUC. It may also be useful in implementation of microsampling techniques as an alternative easier and more patient‐friendly method than the current conventional methods for accurate measurement of tacrolimus, which requires a venipuncture and over 1 ml of blood for each sample drawn.
CONFLICT OF INTEREST
Our group at St. George’s University of London has received research funding and sponsorship for conferences and meetings in relation to nephrology and transplantation from Chiesi, Astellas, Wyeth, Sandoz, and Alexion. Professor Iain MacPhee is currently an employee of AstraZeneca and this work was all undertaken when he was an employee of St. George’s University of London. All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
T.E‐N., I.M., A.J., and J.P. wrote the manuscript. T.E‐N., I.M., A.J., and J.P. designed the research. T.E‐N., I.M., and J.P. performed the research. T.E‐N. and A.J. analyzed the data.
ACKNOWLEDGMENTS
The authos would like to thank clinical research nurses Riny Paul, Sharirose Abat, Jisha Mathew, and Rajeshwar Ramkhelawon for collection of study blood samples at St. George’s University Hospitals NHS Foundation Trust. We also wish to thank the venous access service team, especially Jackie Nicholson, for their assistance in patients’ cannulation at St. George’s University Hospital and Clinical research facility team at St. George’s University Hospital for providing the suitable facilities for blood samples’ collection. The clinical medical and nursing staff at St. George’s Renal and Transplantation Unit for their support in enabling the study take place.
Funding information
The study was supported by an unrestricted research grant (academic funding) from Chiesi LTD. The pharmaceutical company did not play any role in study design, acquisition, and data collection, management, analysis, interpretation, and presentation.
REFERENCES
- 1. Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol. 2007;2:374‐384. [DOI] [PubMed] [Google Scholar]
- 2. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623‐653. [DOI] [PubMed] [Google Scholar]
- 3. Op den Buijsch RAM, van de Plas A, Stolk LML, et al. Evaluation of limited sampling strategies for tacrolimus. Eur J Clin Pharmacol. 2007;63:1039‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Boekel GA, Donders AR, Hoogtanders KE, Havenith TR, Hilbrands LB, Aarnoutse RE. Limited sampling strategy for prolonged‐release tacrolimus in renal transplant patients by use of the dried blood spot technique. Eur J Clin Pharmacol. 2015;71:811‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benkali K, Rostaing L, Premaud A et al. Population pharmacokinetics and Bayesian estimation of tacrolimus exposure in renal transplant recipients on a new once‐daily formulation. Clin Pharmacokinet. 2010;49:683‐692. [DOI] [PubMed] [Google Scholar]
- 6. Barraclough KA, Isbel NM, Kirkpatrick CM, et al. Evaluation of limited sampling methods for estimation of tacrolimus exposure in adult kidney transplant recipients. Br J Clin Pharmacol. 2011;71:207‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mathew BS, Fleming DH, Jeyaseelan V, et al. A limited sampling strategy for tacrolimus in renal transplant patients. Br J Clin Pharmacol. 2008;66:467‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bunnapradist S, Rostaing L, Alloway RR et al. LCPT once‐daily extended‐release tacrolimus tablets versus twice‐daily capsules: a pooled analysis of two phase 3 trials in important de novo and stable kidney transplant recipient subgroups. Transpl Int. 2016;29:603‐611. [DOI] [PubMed] [Google Scholar]
- 9. Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A Steady‐State Head‐to‐Head Pharmacokinetic Comparison of All FK‐506 (Tacrolimus) Formulations (ASTCOFF): an open‐label, prospective, randomized, two‐arm, three‐period crossover study. Am J Transplant. 2017;17(2):432‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaber AO, Alloway RR, Bodziak K, Kaplan B, Bunnapradist S. Conversion from twice‐daily tacrolimus capsules to once‐daily extended‐release tacrolimus (LCPT): a phase 2 trial of stable renal transplant recipients. Transplantation. 2013;96:191‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armendáriz Y, Pou L, Cantarell C, et al. Evaluation of a limited sampling strategy to estimate area under the curve of tacrolimus in adult renal transplant patients. Therapeutic drug monitoring. 2005;71:431‐434. [DOI] [PubMed] [Google Scholar]
- 12. Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503‐512. [DOI] [PubMed] [Google Scholar]
- 13. David OJ, Johnston A. Limited sampling strategies for estimating cyclosporin area under the concentration‐time curve: review of current algorithms. Ther Drug Monit. 2001;23:100‐114. [DOI] [PubMed] [Google Scholar]
- 14. Lee LS, Bertino JS Jr, Nafziger AN. Limited sampling models for oral midazolam: midazolam plasma concentrations, not the ratio of 1‐hydroxymidazolam to midazolam plasma concentrations, accurately predicts AUC as a biomarker of CYP3A activity. J. Clin. Pharmacol. 2006;46:229‐234. [DOI] [PubMed] [Google Scholar]
- 15. Mueller SC, Drewelow B. Evaluation of limited sampling models for prediction of oral midazolam AUC for CYP3A phenotyping and drug interaction studies. Eur J Clin Pharmacol. 2013;69:1127‐1134. [DOI] [PubMed] [Google Scholar]


