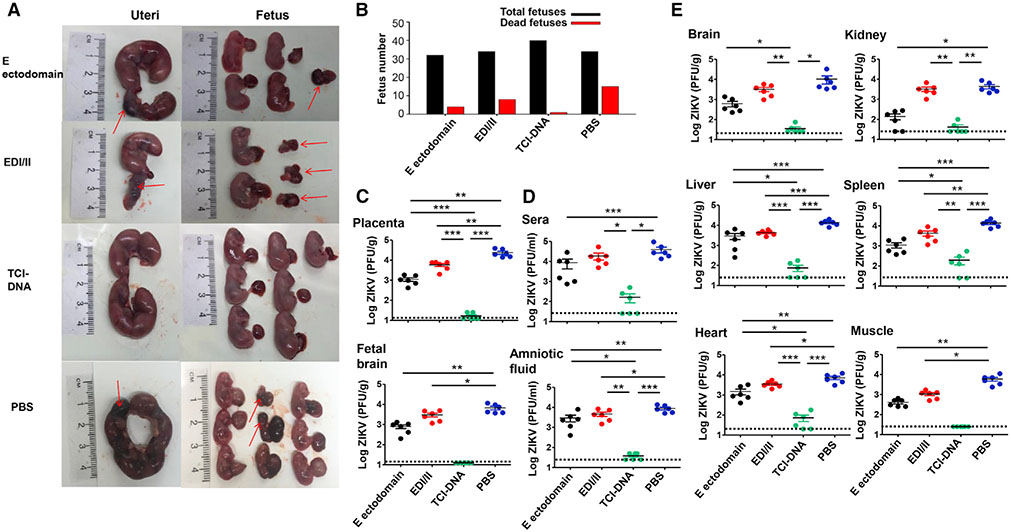
Figure 3. ZIKV TCI-DNA vaccine protected female pregnant Ifnar1−/− mice and their fetuses against ZIKV challenge.
Female Ifnar1−/− mice were immunized with ZIKV E ectodomain, EDI/II peptides, TCI-DNA, or PBS control and mated with male Ifnar1−/− mice for pregnancy at day 10 post-second immunization. The pregnant mice (E10–E12) were challenged with ZIKV (strain R103451, 104 PFU/mouse), and 6 days later, uteri and fetuses were collected to evaluate morphological changes as well as sera, body fluid, and tissues (including placenta) to measure viral titers using plaque-forming assay. Placenta was also observed for apoptosis and vascular damage and examined for inflammatory cytokines and chemokines as described below.
(A and B) Representative image of morphology of uteri (E16–E18) and fetuses from pregnant mice challenged with ZIKV at E10-E12 (A), among which total numbers and dead fetuses from each group are shown (B).
(C–E) Viral titers in placenta, fetal brain (C), as well as sera and amniotic fluid (D) and tissues (including brain, kidney, liver, spleen, heart, and muscle) (E), 6 days post-challenge. The detection limit was 12.5 PFU/g (for placenta and fetal brain), 20 PFU/g (for heart), 25 PFU/ml (for sera and amniotic fluid), and 25 PFU/g (for brain, kidney, liver, spleen, and muscle).
The data are represented as mean ± SEM (n = 6). *p < 0.05; **p < 0.01; ***p < 0.001.