Abstract
Purpose:
To report the cytopathology of vitreous biopsy samples in patients with acute retinal necrosis (ARN) who underwent pars plana vitrectomy (PPV). We also describe two patients with unique clinical courses, cytopathologic findings, and immune response
Methods:
A retrospective review of patients with ARN who developed retinal detachment (RD) and underwent PPV from 2011–2019 at the Emory Eye Center was performed to assess cytopathology findings of vitreous biopsy samples. Patient demographics and laboratory testing including aqueous humor PCR for viral pathogens were recorded. Additional clinical details abstracted included intravitreal injections, surgical procedures, and vitreous cytopathological reports including immunohistochemistry findings.
Results:
Fourteen eyes of twelve patients with RD were reviewed. Ten eyes showed HSV DNA (71%) and 4 demonstrated VZV DNA (29%). All eyes received intravitreal antivirals (i.e. ganciclovir or foscarnet) with a median of 8.5 intravitreal injections per eye. Diagnoses prompting PPV included tractional RD in 14 eyes (100%), rhegmatogenous RD in 8 eyes (57%), vitreous hemorrhage in 4 eyes (29%) and vitreous opacity in 4 (29%). Ophthalmic pathology reports showed lymphocyte populations in 10 eyes (71%) with a CD3+ T-cell predominance in two patients where immunohistochemistry of CD3+ and CD20+ for T- and B-cell populations was performed. Observed immune cell populations included macrophages or histiocytes (11 eyes, 79%) and polymorphonuclear cells in 4 eyes (29%). Initial median VA was 2.5 (IQR 2.0–3.0) and improved to 2.0 (IQR 1.48–3.00, p=0.48) at 6-months and 1.8 (IQR 1.2–3.0, p=0.45) at 12 months follow-up.
Conclusions:
Our cohort of ARN patients undergoing PPV show a spectrum of immunologic findings with the majority demonstrating a lymphocytic response. Histiocytes, macrophages, and PMNs were also observed. Cytopathologic and immunologic studies suggest that both innate and adaptive immunity are responsible for the clinical disease findings observed in ARN. The variability of the response to treatment in patients with ARN may reflect patient-to-patient differences in their antigen-specific immune response. Understanding the immunologic response associated with ARN may provide valuable information regarding the dosing and timing of treatment.
Introduction
Acute retinal necrosis (ARN) is a severe panuveitis syndrome, characterized by granulomatous inflammation, retinal vascular occlusion, and a destructive retinitis.1–3 Patients may progress rapidly to vision loss, often due to optic neuropathy or retinal detachment (RD), particularly if their diagnosis is delayed. ARN was first described by Urayama et al. in 1971 and is typically caused by herpes simplex virus 1 or 2 (HSV-1 or −2, varicella zoster virus (VZV), and rarely cytomegalovirus (CMV) or Epstein-Barr virus (EBV) infection.2,4–5
Following acute infection, severe anterior chamber and vitreous inflammation are observed in association with diffuse or multifocal retinal whitening, with cells rapidly undergoing cytolysis, as the necrotic retina sloughs into the vitreous chamber. The severe vasculitis may also result in retinal artery occlusion or ischemia of the choriocapillaris, driving the inflammatory process further. Confluent retinal necrosis and multiple retinal breaks in the context of vitreous liquefaction and vitreoretinal traction may result in 40% to over 60% of ARN cases being complicated by retinal detachment.6–7 The treatment of ARN thus involves a balance between eradicating the infection with systemic and local antiviral therapy and controlling the inflammatory response without reducing the anti-infective response of the eye.8 Developing a further understanding of the infectious process and the inflammatory response is thus paramount.
In 1982, Culbertson et al described the histopathology of ARN, which showed retinal necrosis, arteritis, and eosinophilic intranuclear inclusions within retinal cells.4 Using electron microscopy, herpesviruses were identified within all layers of affected retinal cells of enucleated eyes. Another study by Rahhal et al described retinal destruction with sloughing of the inner retinal layer, retinal vascular remnants within the vitreous, as well as a mononuclear cell infiltrate in association with ARN due to HSV-2 infection.9 Assessment of the cytokine and chemokine response from ARN patients has shown elevated pro-inflammatory and vascular mediators including IL-6, IL-8, IP-10, Eotaxin, and IL-15 when compared to controls although no obvious TH1 or TH17 pathways were indicated.10
To better understand the immunologic response associated with ARN, we reviewed the cytopathologic findings from vitreous specimens of ARN patients undergoing vitreoretinal surgery. We report the spectrum of inflammatory cells in our cohort of ARN patients, as well as two unique patients with cell populations suggestive of interactions between the adaptive immune response in the inflammatory cascade associated with ARN.
Methods
A retrospective review was performed to assess patients with ARN who developed RD and underwent pars plana vitrectomy (PPV) from 2011 – 2019 at the Emory Eye Center. Emory University Institutional Review Board approval was obtained for this study. All research conformed to the tenets of the Declaration of Helsinki and the Association for Research in Vision and Ophthalmology statement on human subjects research and were compliant with the Health Insurance Portability and Accountability Act of 1996.
Demographic, clinical and surgical history review
Demographic data including age, gender, and time of diagnosis were obtained, as well as follow-up exam data from 6 and 12 month visits. Besides the operative record and pathology findings, the patients’ laboratory testing, viral PCR testing, treatment regimen, intravitreal injections, and pertinent clinical and surgical information were recorded.
Ophthalmic pathology and laboratory testing review
Operative records from vitrectomy surgery and vitreous pathological reports of all patients were reviewed. Specific documentation reviewed included vitrectomy gauge (23- or 25-gauge), clinical indications for surgery (i.e. both initial surgery as well as subsequent surgeries) and operative observations related to the vitrectomy procedure.
All specimens brought to the Montgomery Pathology laboratory were fresh and processed for hematoxylin and eosin staining, as well as PAS staining. Immunohistochemistry for CD68+, CD3+ and CD20+ cells was performed when clinically indicated. Any immunohistochemistry performed by the Ophthalmic Pathology laboratory was reviewed and recorded. Flow cytometry and gene rearrangement studies were also reviewed. Specifically, immunophenotyping for cell surface markers and molecular testing for gene rearrangements were performed in one patient where additional testing was indicated at Emory University Hospital.
Statistical Analysis
Statistical testing was performed with Microsoft Excel (Microsoft, Redmond, WA) and GraphPad Prism (GraphPad Software, San Diego, CA.) Descriptive statistics were reported as medians with interquartile ranges or frequencies with 95% confidence intervals, as appropriate. Visual acuities were converted to logarithm of the minimal angle of resolution (logMAR) as previously described.11 Counting fingers was converted to logMAR of 2.0 while hand motions and was converted to logMAR 3.0.8,12 LogMAR VA of 3.0 was assigned to light perception vision instead of excluding this data, which could otherwise bias the visual acuity to a better median logMAR VA. Wilcoxon signed-rank test for comparison of visual acuity outcomes at the preoperative visit prior to vitrectomy, 6-month visit, and 12-month visit. P-value < 0.05 was considered statistically significant for all analyses.
Results
Baseline demographics, viral etiology, visual acuity outcomes, and surgical indications
Fourteen eyes in 12 patients with ARN who required PPV were identified and their clinical, surgical, pathology, and laboratory data are summarized (Table 1). Two patients demonstrated bilateral involvement (i.e. one patient with bilateral disease at presentation and another patient with bilateral, asynchronous involvement separated by 4 years). One patient had a history of prostate cancer requiring chemotherapy and no patients had a history of HIV-positivity. Ten of 12 patients received oral prednisone with a mean dosage ± standard deviation of 23.0 ± 18.0 mg. There were 6 males (50%) and 6 females (50%) with a median age was 46 (Range 16 – 81 years). All eyes tested positive by PCR testing of their aqueous humor during their management course. Specifically, 10 eyes showed HSV DNA (71)% and 4 eyes demonstrated VZV DNA (29%). All patients received oral or intravenous antiviral therapy and all eyes received intravitreal antivirals with either ganciclovir or foscarnet with a median of 8.5 intravitreal injections per eye (IQR 4–11; Range 1 – 63 injections prior to vitrectomy procedure). The clinical course, visual acuity and RD outcomes of patients 1–6 were summarized previously in a larger cohort recently reported from 2010–2015, but their histopathologic findings and precise surgical course have not been described.13
Table 1.
Demographic and clinical characteristics of surgical ARN cohort
| Variable | Number |
|---|---|
| Total patients (eyes) | 12 (14) |
| Sex | |
| Male (%) | 6 (50) |
| Female (%) | 6 (50) |
| Median age, years (Range) | 46 (16 – 81) |
| Median no. of intravitreal injections* (Range) | 8.5 (1 – 63) |
| PCR testing | |
| Total eyes tested | 14 |
| Total eyes positive | 14 |
| VZV (%) | 2 (14) |
| HSV-1 or 2 (%) | 12 (86) |
Includes ganciclovir or foscarnet
Preoperative findings prompting PPV including tractional RD in 14 eyes (100%), rhegmatogenous RD in 8 eyes (57%), vitreous hemorrhage in 4 eyes (29%) and vitreous opacity in 4 (29%). Two eyes (14%) also had dense cataract requiring pars plana lensectomy at the time of their primary procedure. All eyes had more than one diagnosis prompting surgery.
Initial median preoperative visual acuity was 2.5 (IQR 2.0–3.0) and improved to 2.0 (IQR 1.48–3.00, p=0.48) at 6-months follow-up and 1.8 (IQR 1.2–3.0, p=0.45) at 12 months follow-up, the differences of which were not statistically significant. It is notable, however, that 4 eyes (29%) were better than 20/400 although the majority of eyes (n=10, 71%) demonstrated Snellen visual acuity of poorer than 20/400 at 12-month follow-up.
Ophthalmic pathology findings and analyses
Ophthalmic pathology reports of patients with ARN were reviewed for the cellular components and immune cell populations observed at the time of their first vitrectomy procedure (Table 2). Macrophage and/or histiocyte populations were observed in 11 eyes (79%). Lymphocyte populations were observed in 10 eyes (71%) with a CD3+ T-cell predominance in two patients where immunohistochemistry of CD3+ and CD20+ B-cells was performed. Fibrocellular membranes were observed in 10 eyes (71%), which is consistent with the indication for surgery, which included a majority of patients who had combined tractional and rhegmatogenous components to their RD. Immune cell populations observed also included polymorphonuclear cells in 4 eyes (29%).
Table 2.
Cytopathology findings and iron staining from vitreous fluid
| Pathology findings* | N (%) | 95% CI |
|---|---|---|
| Macrophages / Histiocytes | 11 (79) | 0.49–0.95 |
| Lymphocytes | 10 (71) | 0.41–0.92 |
| Fibrocellular membranes | 10 (71) | 0.41–0.92 |
| Red blood cells | 5 (36) | 0.13–0.65 |
| Polymorphonuclear cells | 4 (29) | 0.08–0.58 |
| Iron staining within macrophages | 4 (29) | 0.08–0.58 |
Total specimens, n = 14 eyes in 12 patients
An iron stain also identified iron-laden macrophages in 4 eyes (29%), all of whom also were found to have clinical evidence of vitreous hemorrhage, admixed with severe inflammatory eye disease. These findings reflected red blood cell degradation products from vitreous hemorrhage and endothelial damage, a common clinical indication for surgery in this cohort of ARN patients. Background debris observed in this series also included erythrocytic debris, retinal fragments, and fibrocellular membrane fragments. Table 3 summarizes the demographic features, clinical indications for surgery, vitrectomy procedures performed, and histopathologic features observed.
Table 3.
Demographic Features, Medical and Surgical Management, Pathology and Visual Acuity
| Pt No. | Age | Sex | Eye | Virus | IVT Med Type (No.) | Procedure(s) | Surgical Indications | Time to first surgery | AC Cell | Vit Cell | Pathology findings during first surgery | Init VA | 6-mo VA | 12-mo VA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | M | OS | HSV | FOS(3); GCV IMP | 1)PPV/MP/SO/EL/FOS 2)PPV/MP/GCV IMP |
1)TRD/RRD/VH 2)TRD/RRD |
1 mo | 1+ | 2–3+ | Mac, LC, PMN, Fe-Mac | HM | HM | HM |
| 2 | 60 | F | OD | HSV | FOS(10)GCV(2) | PPV/MP/SO/EL/FOS | 1)TRD/RRD/VH | 2 mo | 2+ | 2+ | Mac, Fe-Mac, FCM, RBC | HM | LP | LP |
| OS | HSV | FOS(9); GCV(2) | PPV/MP/SO/EL | 1)TRD/VH | 1 mo | 2+ | 2+ | MP, Fe-Mac, FCM, RBC | HM | LP | LP | |||
| 3 | 47 | F | OS | HSV | FOS(7); GCV(1) | 1) PPV/SB/SO/EL/FOS 2) PPV/PPL/MP/SO 3) PPV/PPL 4) PPV/MP/EL/SO |
1)TRD/RRD 2)TRD/PVR/Cat 3)TRD/ERM 4)PVR |
1 mo | 1–2+ | 2–3+ | LC, PMNs, HIS, FCM | CF | CF | CF |
| 4 | 63 | F | OD | HSV | FOS(1) | 1) PPV/EL/SO 2) PPV/ROSO/EDTA |
1)TRD/VH 2)TRD/SO/Band K |
2 wks | Rare | 2–3+ | FCM, RBC, Fe-Mac | HM | CF | HM |
| 5 | 20 | M | OD | HSV | FOS(45),GCV(18) | 1)PPV/MP/EL/SO 2)PPV/MP/EL/SO 3)PPV/ROSO 4)PPV/ROSO/EDTA |
1)TRD/RRD 2)TRD/RRD/VO 3)PVR 4)TRD/Band K |
5 mo | Rare | 3+ | LC*, FCM, Mac | 20/200 | 20/400 | 20/200 |
| 16 | OS | HSV | FOS(3) | 1)PPV/PPL/MP/SO/EL/FOS | 1)TRD/RRD/Cat | 1 mo | 2–3+ | 2–3+ | LC, HIS, PMNs, FCM | HM | LP | LP | ||
| 6 | 81 | M | OD | VZV | FOS(8) | PPV/SB/EL/SO | TRD/RRD/VO | 3 mo | Rare | 0.5 | LC, HIS, FCM | LP | 20/200 | 20/300 |
| 7 | 36 | F | OD | HSV | FOS(3) | 1)PPV/MP/EL/FAX/FOS 2)PPV/MP/EL/SO/FOS |
1)TRD/VO 2)TRD/VO |
2 wks | 3+ | 3–4+ | LC, FCM | CF | HM | HM |
| 8 | 71 | M | OD | HSV | FOS(6);GCV(1) | PPV/MP/EL/SO/FOS | TRD/RRD | 3 wks | 0.5 | 2+ | LC, HIS | 20/400 | 20/600 | 20/125 |
| 9 | 38 | F | OD | VZV | FOS(7); GCV(3) | PPV/MP/EL/SO | TRD/RRD | 1 mo | 1+ | 1+ | Mac, LC**, PMN, RBC | 20/800 | 20/600 | 20/200 |
| 10 | 46 | F | OD | HSV | FOS(11) | 1) PPV/MP/EL/SO 2) PPV/MP/EL/SO |
1)TRD/VO 2)TRD/RRD/PVR/SRM |
2 mo | 0.5+ | 2+ | FCM, LC | CF | CF | 20/800 |
| 11 | 29 | M | OD | HSV | FOS(7); GCV(5) | PPV/MP/EL/SO | TRD/VO | 3 wks | 1–2+ | 1–2+ | LC, HIS, PMN | HM | 20/500 | 20/500 |
| 12 | 23 | M | OD | HSV | FOS(9) | 1)PPV/PPL 2)PPV/EDTA |
1) TRD/Cat 2) TRD/Band K |
2 mo | 3+ | 2+ | Lens, RBC, FCM | CF | CF | LP |
Abbreviations
F Female, M Male, OD Right eye, OS Left eye, FOS Foscarnet, G Ganciclovir
PPV Pars plana vitrectomy, MP Membrane peel, PPL Pars plana lensectomy, EL Endolaser, SO silicone oil, EDTA Ethylenediamine tetraacetic acid chelation, SB Scleral buckle, ROSO Removal of silicone oil
TRD Tractional retinal detachment, RRD Rhegmatogenous retinal detachment, PVR Proliferative vitreoretinopathy, Band K Band Keratopathy, VH Vitreous hemorrhage, VO Vitreous opacity, SRM Subretinal membranes, Cat Cataract
Mac Macrophages, LC Lymphocytes, PMN Polymorphonuclear cells (Neutrophils), FCM Fibrocellular membranes, RBC Red blood cells/erythrocytes, Fe-Mac Iron-laden macrophages
HM Hand motions, CF Counting fingers, LP Light perception
Flow cytometry of vitreous fluid in patient 5 showed predominantly T-cells
Lymphocytes described as largely CD3+ with scattered CD20+ lymphocytes
Representative cases with detailed cytopathologic and molecular analysis
In this cohort of patients undergoing surgery for ARN, two patients had particularly unique clinical courses and pathologic findings for which additional detailed clinical and immune phenotyping were performed. In one patient, a lymphocyte predominance was observed and raised concerns for a lymphoproliferative process and in a second patient, severely recalcitrant disease was assessed by cytopathology and immunohistochemistry for B- and T-cell populations. Their clinical course, pathologic features, and additional molecular and laboratory diagnostics are summarized.
Case 1
A 38-year-old female patient (Patient 9) presented with 20/800 vision in the right eye, a 2+ afferent pupillary defect with severe restriction of her visual fields by confrontation. Slit lamp examination showed 3+ anterior chamber (AC) cell and 1–2+ vitreous haze. Fundus photography showed optic disc edema, sheathing and hemorrhage along the major vessels, as well as peripheral retinal whitening. Fundus exam of the left eye showed dense chorioretinal scar, which had previously been attributed to toxoplasmosis chorioretinitis (Figure 1). Her AC tap of the right eye was positive for HSV PCR and negative for toxoplasmosis, varicella zoster virus, and cytomegalovirus PCR. She required 10 total intravitreal injections and developed a RD 1 month after presentation, prompting RD repair with vitrectomy, membrane peel and silicone oil instillation.
Figure 1.
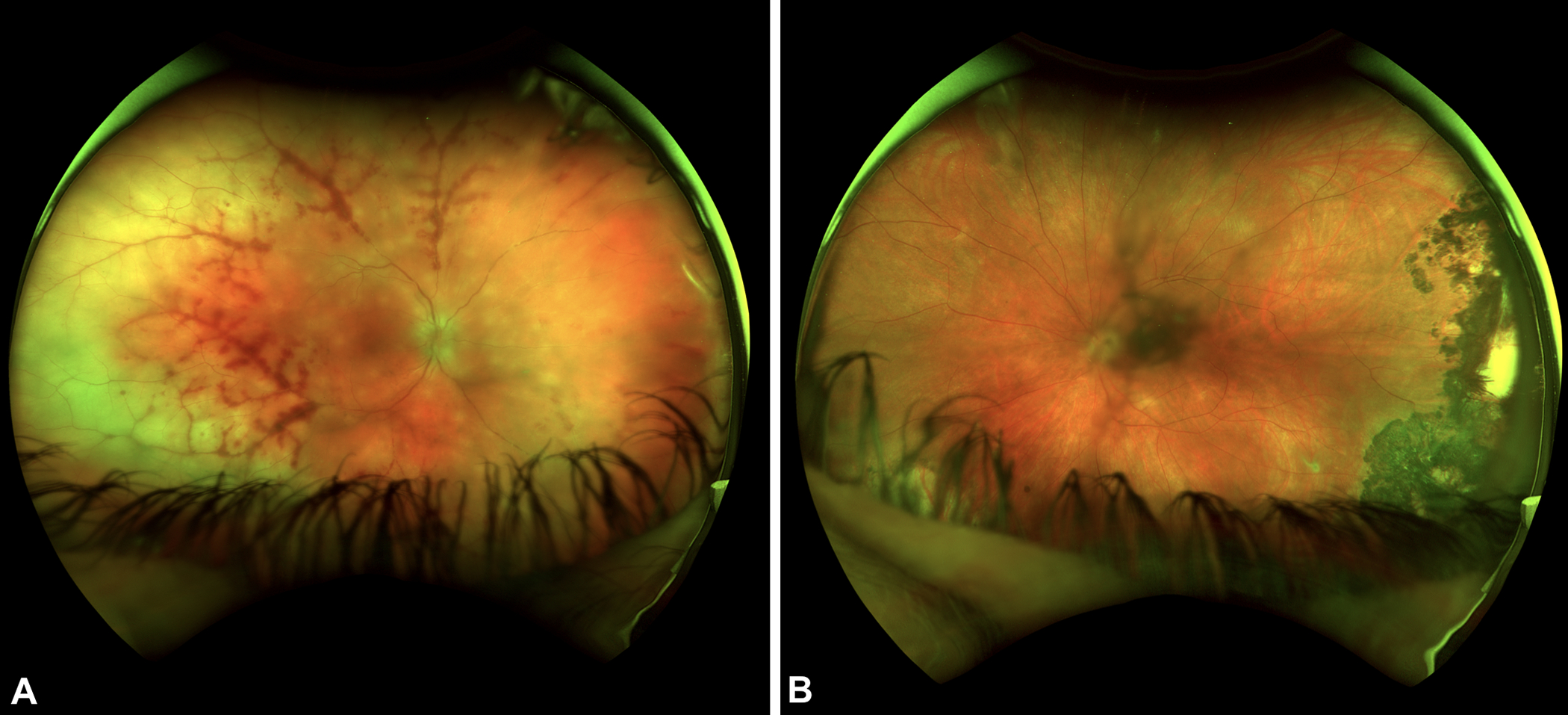
A) Ultra-widefield fundus photographs of patient 9 show diffuse peripheral retinal whitening temporally greater than nasally associated with severe perivascular hemorrhage. There are also multifocal areas of whitening in the peripapillary region and optic disc edema of the right eye. B) Ultrawide-field fundus photograph of the left eye shows a dense hyperpigmented chorioretinal scar temporally. She had a clinical diagnosis of toxoplasmosis previously but the extent of scarring suggests she may have had HSV ARN in the left eye previously.
The patient’s vitreous biopsy showed a highly cellular appearance, consisting of a dense lymphocytic infiltrate and mononuclear cells with a high nuclear-to-cytoplasmic ratio. The presence of atypical cells on biopsy prompted immunohistochemistry staining, showing a large number of CD68+ as well as CD3+ T-cells, with scattered CD20+ B-cells (Figure 2). Of note, there was a lack of tingible body macrophages and apoptotic large lymphocytes, which may be seen in vitreoretinal lymphoma. Additionally, IgH and kappa gene rearrangement testing revealed a clonal gene rearrangement, which was initially concerning for lymphoma and prompted Neuro-oncology consultation. Magnetic resonance imaging scan of the brain was performed and showed no lesions or evidence of lymphoma. A lumbar puncture was performed and cerebrospinal fluid analysis showed CD3+ T-cells and no CD19+ B-cell lymphocytes. Given that there was no evidence of central nervous system lymphoma, no systemic intervention was recommended. The patient’s ophthalmic examination showed dense retinal whitening and exudation but she continued to improve with antiviral therapy and the retinal whitening eventually resolved with no recurrences while on antiviral therapy. Based on the clinical response, the patient’s monoclonal B-cell proliferation was deemed to be most consistent with a reactive, monoclonal population among a predominantly inflammatory T-cell population.
Figure 2.
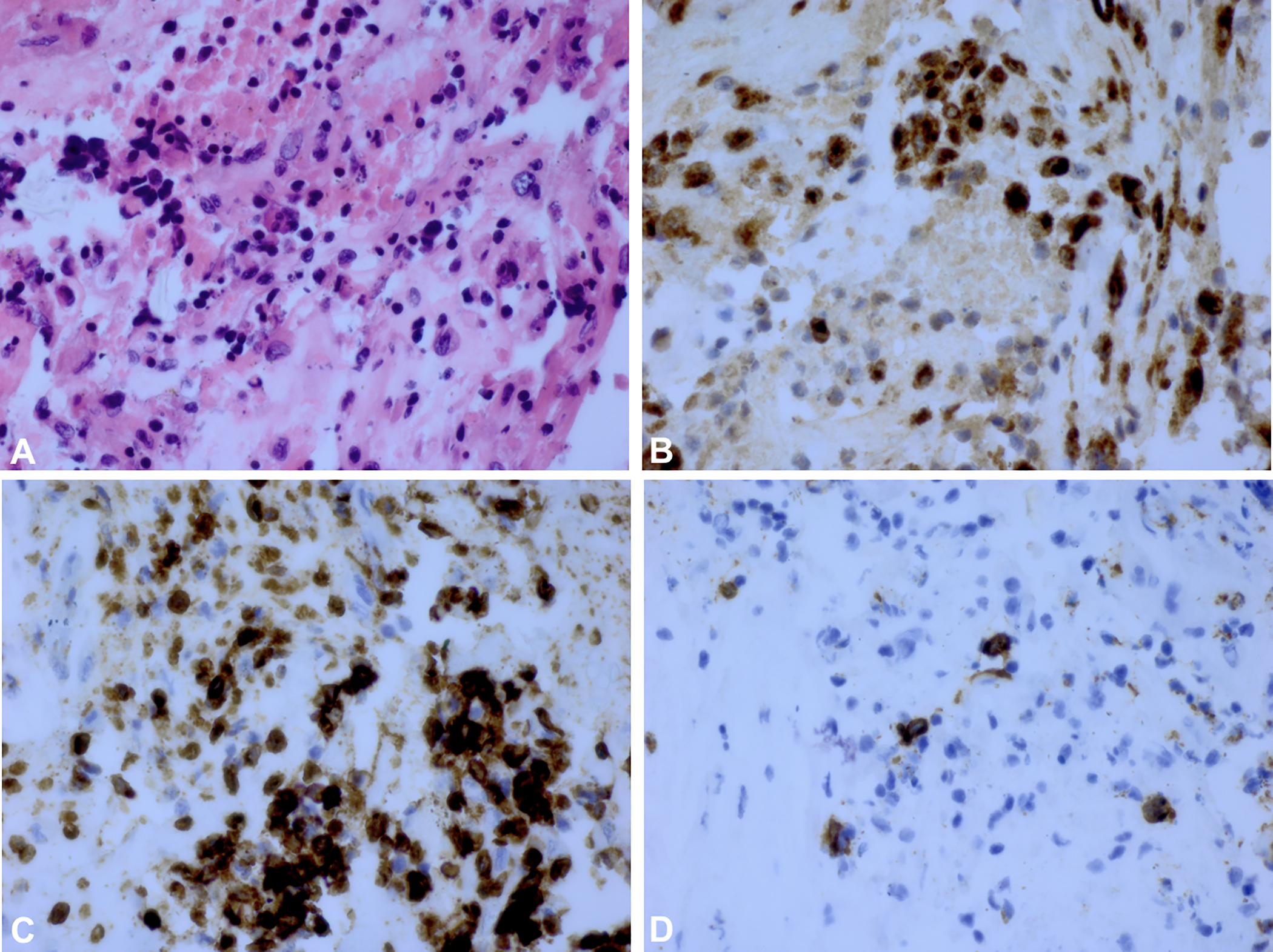
A) H&E stain of vitreous biopsy for Case 1 (Patient 9) with HSV-2 associated ARN. B) Staining for CD68+ cells show a large number of macrophages in brown. C) The brown colored areas show a predominance of CD3+ T-cells. D) Staining for CD20+ B-cells shows scattered B-cells in brown suggesting a B-cell population within a predominantly T-cell lymphocyte population.
The patient’s eventual ophthalmic disease resolution and the lack of systemic or CNS lymphoma at long-term follow-up allayed concerns that the clinical and pathologic findings were related to lymphoma. No evidence of CNS lymphoma or disease progression was observed at 12-months follow-up.
Case 2
A 16-year-old male (Patient 5) presented with hand motion visual acuity and a dense RAPD in the left eye. Slit lamp examination was notable for 2+ AC cell and there was a dense vitritis precluding a view to the posterior pole. His aqueous humor tested positive for HSV-2 by PCR, and ARN associated with HSV-2 was diagnosed. The patient was treated with valacyclovir, oral corticosteroid and 3 intravitreal foscarnet injections for ARN. Two weeks later, a combined tractional and rhegmatogenous retinal detachment was diagnosed by B-scan ultrasound, prompting PPV for the left eye. After four months, he had a recurrent total RD and underwent a second vitrectomy procedure, but his visual acuity progressively declined from hand motions to light perception vision at 12 months follow-up. Pathologic evaluation of the vitreous contents showed lymphocytes, histiocytes and fibrocellular membranes.
The patient was lost to follow-up for 2 years and his oral antiviral had been discontinued. He then presented with subacute onset of blurred vision in the right eye and his exam was consistent with ARN with diffuse, severe retinitis in the previously unaffected eye (Figure 3). An anterior chamber paracentesis was sent for PCR and tested positive for HSV-2 DNA. Given his monocular status and severity of disease, the patient was hospitalized for parenteral acyclovir and serial intravitreal injections. His intravitreal regimen consisted of combination ganciclovir/foscarnet, altering with foscarnet injections every 3 days and he was closely monitored for disease resolution. After 6 weeks in the hospital and disease stability was observed, the patient was discharged with oral valacyclovir 1 gram TID and weekly intravitreal foscarnet in consultation with Infectious Diseases and Hospital Medicine services. The patient eventually required 45 intravitreal foscarnet injections and 18 ganciclovir injections to achieve disease resolution. Six months after his initial presentation, his clinical course was complicated by a superonasal retinal detachment, which required PPV, endolaser, and silicone oil tamponade. The patient eventually underwent three additional vitrectomy procedures for recurrent RD. After 12 months of follow-up, the patient’s visual acuity had improved to 20/200 and his exam findings were stable with no evidence of ARN recurrence.
Figure 3.
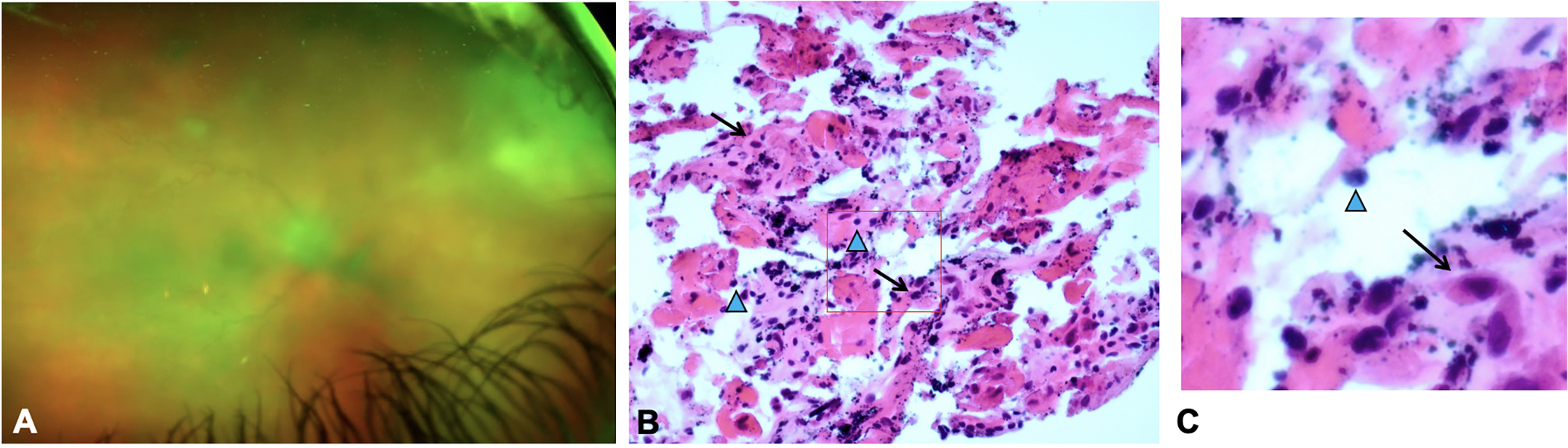
Clinical photograph of case 2 (Patient 5) shows 2+ vitreous haze and dense retinitis in the superonasal quadrant (A). Following multiple intravitreal injections, PPV was performed. H&E stain of vitreous biopsy from Case 2 (Patient 5) shows cellular inflammation in HSV-2 associated ARN (B). Cytopathology showed fibrocellular membranes, lymphocyte populations (blue arrowheads) and macrophages (arrows). A higher magnification inset highlights the lymphocytes and macrophage cell populations (C). Because this biopsy shows less cellularity and absence of atypical cells, his case was not concerning for lymphoma. CD3+ and CD68+ staining showed predominantly T-cell population of lymphocytes (not shown).
The patient’s pathologic findings showed a severe inflammatory response with a mixed population of immune cells including neutrophils, B- and T-lymphocyte cell populations, as well as fibrocellular membranes (Figure 3).
Conclusion
In this cohort of ARN patients who underwent vitreoretinal surgery, a severe inflammatory cell reaction was observed within vitreous specimens in which lymphocytes, macrophages or histiocytes and fibrocellular membranes predominated. Neutrophils were also observed, often with iron-laden macrophages in the eyes with vitreous hemorrhage. Our interest in the histopathology of ARN was sparked by the index patient (Patient 9) with HSV-ARN who showed a monoclonal B-cell proliferation within an inflammatory cell population. This prompted further exploration of vitreous biopsy findings in additional cases of ARN complicated by RD requiring PPV.
In our index case, a monoclonal proliferation of B-cells was observed by flow cytometry and gene rearrangement. A detailed clinical neuro-oncology and molecular workup led to the impression that this cell population was reactive, lying within a predominantly inflammatory T-cell population based on CD3+ immunohistochemical staining. The patient’s clinical phenotype was particularly unique in that a large area of retinal whitening was difficult to treat despite aggressive antiviral therapy. This area of retinal whitening was also associated with severe retinal exudation and lipid deposition that resolved over time.
While innate immunity likely played a role in disease pathogenesis, given the findings of reactive histiocytes and PMNs, acquired immunity was also implicated given that the majority of patients showed lymphocytic infiltration of the vitreous cavity. Vitreous fluid analysis showed a monoclonal expansion and initially raised concerns about a neoplastic process. While it is important to note that clonality does not equate to malignancy, this relationship of immunologic reaction and virus response could also explain the recalcitrant nature of persistent retinal whitening and ongoing necrosis despite aggressive intravitreal antiviral therapy.
In the second case described in detail, the patient developed bilateral, sequential ARN involvement over a span over 4 years and his disease was particularly recalcitrant, requiring over 60 intravitreal injections over an approximately 6-month follow-up period to achieve disease resolution. We hypothesize that ongoing severe retinal inflammation, triggered by disease reactivation may have contributed to his disease phenotype, making our combination of systemic and intravitreal antiviral therapy less effective in disease resolution. High-dose parenteral corticosteroid and corticosteroid injections were considered but ultimately deferred given the patient’s monocular status and concern that corticosteroid therapy could dampen the antiviral immune response and lead to more severe and recalcitrant disease. Indeed, severe, recalcitrant disease with poor visual outcomes has been observed in ARN patients following corticosteroid administration without antiviral therapy.14–15
Prior reports have evaluated enucleated specimens and cytokine profiling in patients4,10, but histopathology in vivo has not been characterized fully. Our series provides additional insight on the range of immunologic responses in ARN. Understanding the immunologic milieu, antigen-specific acquired immunity and innate immunity would help our understanding of treatment paradigms including use of corticosteroid or other anti-inflammatory medications.
Animal models studying ARN have provided insight into the immune pathogenesis of ARN. Specifically, Zheng et al investigated infiltrating immune cells and the kinetics of cytokine expression in a murine model of HSV-1-induced ARN. They found that following inoculation of HSV-1 into the anterior chamber of BALB/c mice, the uninoculated, contralateral eye that developed ARN showed CD4+ T-cells, F4/80+ macrophages, PMNs and CD19+ B-cells. Pro-inflammatory cytokines including TNF-alpha, IFN-gamma, and IL-4 were also upregulated in affected, contralateral eyes and attributed to infiltrating immune cells and retinal cells.16
Interestingly, earlier work by Streilein et al in this murine model for ARN in athymic mice provide further evidence of antigen-specific T-cell response in the pathogenesis of ARN. Specifically, following uniocular anterior chamber injection of HSV-1 (KOS strain) in BALB/c and A/J mice, ARN develops in the contralateral, uninjected eyes only in mice that are immunocompetent; athymic mice show HSV-1 titers in the contralateral eye without evidence of ARN. In vitro evaluation of ipsilateral cervical lymph nodes of immunocompetent mice with ARN show T-cells that proliferate in response to HSV antigen and express IL-2 receptors. Further studies have shown that the infiltration of CD4+ and CD8+ T-cell coincides with the onset of retinal necrosis and not the peak of virus replication – the presence of these immune cell populations support their key role in perpetuating the clinical findings of ARN.17
Considering the spectrum of immune cells observed in vitreous specimens of our patients and immune cell milieu observed in animal models, it is plausible that while viral reactivation and lytic infection are key initiating events, both innate and adaptive immunity are responsible in the severe disease phenotype often observed. Two patients with severe, diffuse retinal whitening that was recalcitrant to therapy suggest that antiviral medications, while effective in dampening viral replication, were ineffective at rapidly clearing the inflammatory disease process.
Limitations of this study include case selection, as all patients underwent vitreoretinal surgery for RD repair, which introduces bias for more severe cases. The majority of our patients showed fibrocellular elements and lymphocytes, associated with a severe vitreous inflammatory response and multiple necrotic retinal breaks. We have previously described that poorer visual acuity at disease onset is associated with a greater risk for RD development, possibly due to the role of greater vitreous inflammation and immune cell recruitment.13
In summary, the vitreous specimens in our cohort of ARN patients undergoing RD repair show a spectrum of immunologic findings characterized by lymphocytic response with a significant minority of patients with PMN and histiocytic involvement. The variability of the response to treatment in patients with ARN may reflect patient-to-patient differences in their antigen-specific immune response. Two patients in this series were particularly refractory to therapy including one patient with a monoclonal B-cell population that supports this notion. Further studies regarding the timing of innate and acquired immune cell responses, associated cytokine responses, and the relationship of immunologic response to viral load may provide valuable information regarding appropriate dosing of antiviral medication and the timing of corticosteroid or alternative anti-inflammatory medications.
Funding
This project was supported by the National Eye Institute/National Institutes of Health core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine), National Eye Institute of the National Institutes of Health under award number K23 EY030158 (Shantha) and R01 EY029594 (Yeh). This research was also supported an unrestricted departmental grant from Research to Prevent Blindness, Inc. to the Emory Eye Center, Emory University School of Medicine, the Bayer Global Ophthalmology Awards Program, Association for Research in Vision and Ophthalmology Mallinckrodt Young Investigator Award (SY), and National Institutes of Health funding for the Emory Center for AIDS Research (Kraft, P30AI050409). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Prior Presentation: This data was presented in part at the American Uveitis Society meeting at the American Academy of Ophthalmology Annual Meeting in San Francisco, CA, October 2019.
References
- 1.Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol 1994. May; 117(5): 663–7. [DOI] [PubMed] [Google Scholar]
- 2.Duker JS, Blumenkranz MS. Diagnosis and management of the acute retinal necrosis (ARN) syndrome. Surv Ophthalmol. 1991. Mar-Apr;35(5):327–43. [DOI] [PubMed] [Google Scholar]
- 3.Shantha JG, Weissman HM, Debiec MR, et al. Advances in the management of acute retinal necrosis. Int Ophthalmol Clin 2015;55:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culbertson WW, Blumenkranz MS, Haines H, et al. The acute retinal necrosis syndrome. Part 2: Histopathology and etiology. Ophthalmology 1982;89:1317–25. [Crossref] [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Urayama A, Yamada N, Sasaki T. Unilateral acute uveitis with periarteritis and detachment. Jpn J Clin Ophthalmol 1971;25:607–19. [Google Scholar]
- 6.Baltinas J, Lightman S, Tomkins-Netzker O. Comparing treatment of acute retinal necrosis with either oral valacyclovir or intravenous acyclovir. Am J Ophthalmol 2018. Apr; 188:173–80. [DOI] [PubMed] [Google Scholar]
- 7.Kopplin LJ, Thomas AS, Cramer S, et al. Long-term surgical outcomes of retinal detachment associated with acute retinal necrosis. Ophthalmic Surg Lasers Imaging Retina 2016. Jul; 47(7): 660–4. [DOI] [PubMed] [Google Scholar]
- 8.Flaxel CJ, Yeh S, Lauer AK. Combination systemic and intravitreal antiviral therapy in the management of acute retinal necrosis syndrome (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2013. Sep; 111: 133–44. [PMC free article] [PubMed] [Google Scholar]
- 9.Rahhal FM, Siegel LM, Russak V, et al. Clinicopathologic correlations in acute retinal necrosis caused by herpes simplex virus type 2. Arch Ophthalmol 1996. Nov; 114(11): 1416–9. [DOI] [PubMed] [Google Scholar]
- 10.De Visser L, de Boer JH, Rijkers GT, et al. Cytokines and chemokines involved in acute retinal necrosis. Invest Ophthalmol Vis Sci 2017. Apr; 58(4): 2139–51. [DOI] [PubMed] [Google Scholar]
- 11.Holladay JT. Proper method for calculating average visual acuity. J Refractive Surgery 1997. July-Aug; 13(4):388–91. [DOI] [PubMed] [Google Scholar]
- 12.Tibbetts MD, Shah CP, Young LH, et al. Treatment of acute retinal necrosis. Ophthalmology 2010. Apr; 117(4): 818–24. [DOI] [PubMed] [Google Scholar]
- 13.Debiec MR, Lindeke-Myers AT, Shantha JG, et al. Outcomes of combination systemic and intravitreal antiviral therapy for acute retinal necrosis. Ophthalmol Retina 2020. Jul; S2468–6530. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissman HM, Biousse V, Schechter MC, et al. Bilateral central retinal artery occlusion associated with herpes simplex virus-associated acute retinal necrosis and meningitis: case report and literature review. Ophthalmic Surg Lasers Imaging Retina 2015;46:279–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh S, Fahle G, Flaxel CJ, Francis PJ. Central retinal vascular occlusion associated with acute retinal necrosis. Arch Ophthalmol 2012. Apr; 130(4)514–7. [DOI] [PubMed] [Google Scholar]
- 16.Zheng M, Atherton SS. Cytokine profiling and inflammatory cells during HSV-1-induced acute retinal necrosis. Invest Ophthalmol Vis Sci 2005. Apr; 46(4): 1356–63. [DOI] [PubMed] [Google Scholar]
- 17.Streilein JW, Igietseme JU, Atherton SS. Evidence that precursor cytotoxic T cells mediate acute necrosis in HSV-1-infected retinas. Curr Eye Res 1991; 10 Suppl: 81–6. [DOI] [PubMed] [Google Scholar]


