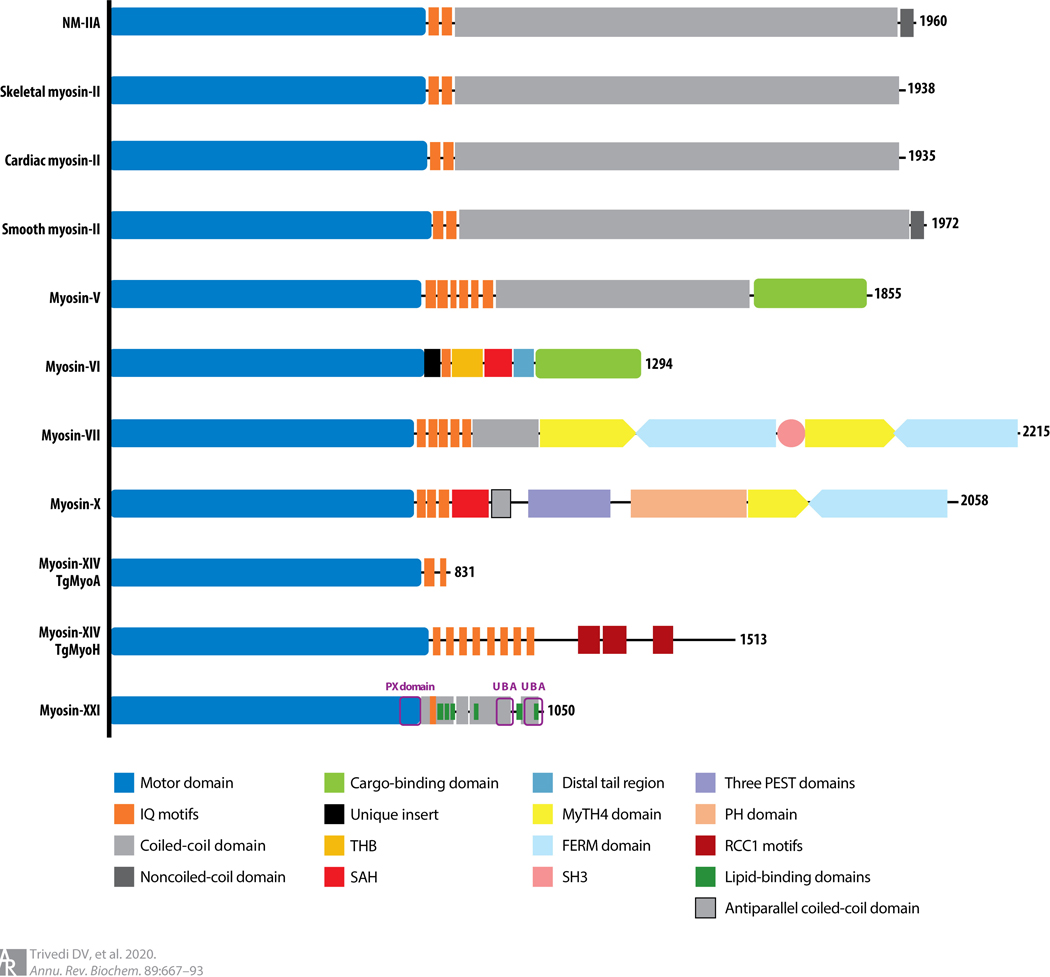
Figure 1.
Linear structure depictions of representative members of the myosin family of molecular motors. The N-terminal domain is a globular motor domain (blue), followed generally by one or more IQ motifs (dark orange) that bind either calmodulin molecules or specialized light chains similar in size to calmodulin. Myosin-VI has a unique insert (black) between the motor domain and the IQ domain that redirects the motor movement toward the minus end of the actin filament. Many myosins are dimeric owing to the assembly of two α-helices into a coiled coil (gray). Other specialized domains are highlighted by different colors: THB (light orange), SAH (red), and others as listed in the key. Abbreviations: FERM, 4.1 protein, ezrin, radixin, moesin; IQ, isoleucine-glutamine; MyTH4, myosin tail homology 4; NM-IIa, nonmuscle myosin-IIa; PEST, sequence rich in proline, glutamic acid, serine, and threonine residues; PH, pleckstrin homology; PX, phox homology; RCC1, related regulator of chromosome condensation 1; SAH, single α-helix; SH3, Src homology region type 3; TgMyo, Toxoplasma gondii myosin; THB, three-helix bundle; UBA, ubiquitin-associated.