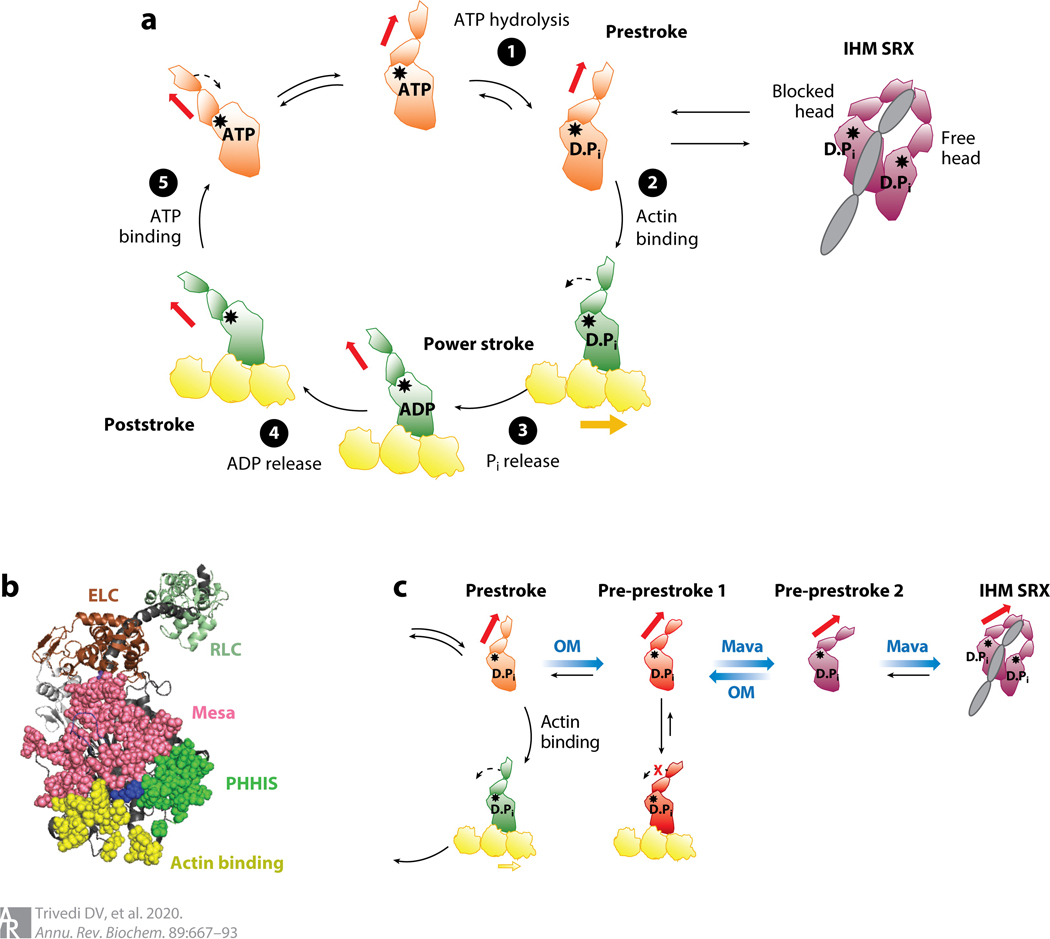
Figure 4.
S1 heads of myosin-II in various states. (a) Chemomechanical cycle of the interaction of myosin heads with actin. ❶ The S1, free of actin, hydrolyzes ATP, and the products remain bound to the active site. ❷ The prestroke S1 with bound ADP and Pi binds to actin (yellow). ❸ While bound to actin, associated with Pi release, the lever arm swings to the left (black dashed arrow) about a fulcrum point (black star), moving the actin filament to the right (bold yellow arrow) with respect to the myosin thick filament.❹ ADP release allows a further small stroke to the poststroke position and frees the active site for binding of ATP. ❺ ATP binding weakens the interaction of the S1 with actin. The prestroke state can be pulled into the folded IHM SRX state (purple). Actin-bound and actin-unbound myosin heads are shown in green and orange, respectively. Red arrow denotes direction of the lever arm. (b) PyMOL structure showing three critical surfaces of the S1: the actin-binding face (yellow), the mesa (pink), and the PHHIS (green). At the apex of the pyramid formed by these three surface domains is residue Arg403 (blue), which, when mutated to a glutamine, causes hypertrophic cardiomyopathy. The ELC (brown) and RLC (pale green) are shown attached to the lever arm heavy-chain α-helix (dark gray). This homology model of the prestroke state of human β-cardiac S1 can be downloaded from our website (http://spudlab.stanford.edu/homology-models). (c) Predicted different conformational states for OM-bound (red) and Mava-bound (purple) S1 heads. Abbreviations: D.Pi, ADP.Pi; ELC, essential light chain; IHM, interacting heads motif; Mava, mavacamten; OM, omecamtiv mecarbil; PHHIS, primary head-head interaction site; Pi, inorganic phosphate; RLC, regulatory light chain; S1, subfragment 1; SRX, super relaxed.