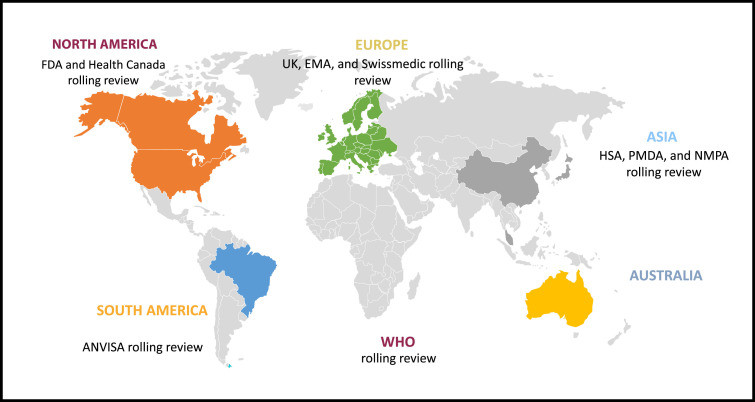
Figure 1.
Pictogram showing the health authorities around the globe that introduced or offered a rolling review process to applicants for the evaluation of COVID-19 treatments and vaccines. ANVISA = Brazilian Agencia Nacional de Vigilancia Sanitaria; EMA = European Medicines Agency; FDA = US Food and Drug Administration; HAS = Health Science Authority; MHRA = UK Medicines and Healthcare Products Regulatory Agency; NMPA = Chinese National Medical Products Administration; PMDA = Pharmaceuticals and Medical Devices Agency; WHO = World Health Organization.