Abstract
At present, growing evidence indicates that long non‐coding RNAs (lncRNAs) participate in the progression of glioma. The function of LOXL1‐AS1 in vasculogenic mimicry (VM) in glioma remains unclear. First, the expressions of TIAR, the lncRNA LOXL1‐AS1, miR‐374b‐5p and MMP14 were examined by qRT‐PCR and Western blot in both, glioma tissues and glioma cell lines. Proliferation, migration, invasion and tube formation assays were conducted to evaluate the roles of TIAR, LOXL1‐AS1, miR‐374b‐5p and MMP14 in malignant cellular behaviours in glioma cells. A nude mouse xenograft model and dual staining for CD34 and PAS were used to assess whether VM was affected by TIAR, LOXL1‐AS1 or miR‐374b‐5p in vivo. In this study, low levels of TIAR and high levels of LOXL1‐AS1 were found in glioma cells and tissues. TIAR downregulated the expression of LOXL1‐AS1 by destabilizing it. LOXL1‐AS1 acted like a miRNA sponge towards miR‐374b‐5p so that downregulation of the former greatly inhibited cell proliferation, migration, invasion and VM. Additionally, miR‐374b‐5p overexpression repressed malignant biological behaviours and VM in glioma by modifying MMP14. In summary, we demonstrated that TIAR combined with LOXL1‐AS1 modulates VM in glioma via the miR‐374b‐5p/MMP14 axis, revealing novel targets for glioma therapy.
Keywords: glioma, LOXL1‐AS1, miR‐374b‐5p, MMP14, TIAR, vasculogenic mimicry
1. INTRODUCTION
Glioma is commonly acknowledged as one of the most malignant tumours of the central nervous system and is characterized by high mortality and low survival rates. 1 Despite the development of various surgeries and medicines over the years, the prognosis of glioma patients remains poor. 2 , 3 , 4 Therefore, exploring new targets for glioma therapy should be prioritized.
Currently, anti‐angiogenesis therapy is becoming increasingly attractive to surgeons worldwide. However, for some reason, the prognosis has not improved much with anti‐vascular endothelial cell therapy. Vasculogenic mimicry (VM), a newly discovered form of angiogenesis in which vessels are surrounded by tumour cells rather than vascular endothelial cells, is now gaining attention. 5 Several reports have discovered VM in diverse cancers, such as glioma, hepatocellular carcinoma, gastric cancer, non–small‐cell lung cancer and colorectal cancer. 6 , 7 , 8 , 9 , 10 VM has a close association with tumour metastasis and is a predictor of poor clinical prognosis. 11 , 12
RNA‐binding proteins (RBPs) participate in tumour development, playing roles in pre‐mRNA splicing, translation and RNA stabilization. 13 Increased TIA1‐related protein (TIAR) expression represses the proliferation of 293 cell lines and xenograft tumour growth. 14 Additionally, TIAR could prolong the survival of patients with astrocytoma and glioblastoma multiforme, while G3BP1 has the opposite effect. 15 However, the expression of TIAR in glioma and its possible association with progression have not yet been reported.
Long non‐coding RNAs (lncRNAs) are non‐coding RNAs >200 nucleotides in length. Growing evidence shows that lncRNAs play a vital role in oncogenesis and tumour growth. LOXL1 antisense RNA1 (LOXL1‐AS1) downregulates miR‐708‐5p and promotes malignant behaviour in breast cancer. 16 Additionally, LOXL1‐AS1 participates in regulating drug resistance in prostate cancer by modifying miR‐let‐7a‐5p and EGFR. 17 Furthermore, LOXL1‐AS1 exerts an oncogenic role in laryngocarcinoma, ovarian cancer and colorectal cancer. 18 , 19 , 20 Nevertheless, the mechanism of action of LOXL1‐AS1 in glioma, especially in VM, is elusive.
MicroRNAs (miRNAs) participate in the post‐transcriptional processes of tumorigenesis by regulating the 3′‐UTR of downstream target genes. miR‐374b‐5p downregulates ABCA8 and promotes carcinogenesis in hepatocellular carcinoma. 21 Moreover, MiR‐374b‐5p plays an antitumour role in pancreatic and cervical cancers. 22 , 23 Nevertheless, we know little about miR‐374b‐5p expression and its function in VM in gliomas.
Matrix metalloproteinase 14 (MMP14), a member of the membrane‐type MMP family, is strongly associated with tumour metastasis. MMP14 also plays a VM‐related role in gastric carcinoma, hepatocellular carcinoma and lung cancer. 24 , 25 , 26
This study aimed to investigate the expressions of TIAR, LOXL1‐AS1 and miR‐374b‐5p in both, glioma tissues and glioma cells. Further, the functions of TIAR, LOXL1‐AS1, miR‐374b‐5p and MMP14, and their interactions in modulating cellular behaviours and VM in glioma are yet to be established. Our study provides new potential therapeutic targets for glioma therapy.
2. MATERIALS AND METHODS
2.1. Clinical specimens
We obtained 37 tissues in total, according to the WHO classification of central nervous system tumours, including 13 low‐grade glioma tissues (LGGTs; WHO I‐II), 16 high‐grade glioma tissues (HGGTs; WHO III‐IV) and 8 normal brain tissues (NBTs). All tissues were from material discarded during surgery for glioma or traumatic brain injury. Ethical approval was obtained for the current research.
2.2. Cell culture
Normal human astrocytes (NHA) along with HEK293T, U87 and U251 cell lines were purchased from the Shanghai Cell Bank affiliated to the Chinese Academy of Life Sciences. NHA were incubated in 1640 medium, while the HEK293T, U87 and U251 cells were incubated in high‐glucose medium with 10% foetal bovine serum (Gibco). The growth conditions were 37°C and 5% CO2, together with a certain degree of humidity.
2.3. Quantitative real‐time PCR (qRT‐PCR)
After the total RNA was extracted according to the manufacturer's manual of Trizol reagent, TIAR, LOXL1‐AS1, MMP14 and GAPDH expressions were determined using SYBR One Step RT‐PCR kits (Takara). The detections of miR‐374b‐5p and U6 were performed using the miRNA First Strand cDNA Synthesis kit and the MicroRNA qPCR Kit (Sangon Biotech,). All primers were designed by Sangon Biotech and are presented in Table S1.
2.4. Western blot analysis
After electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes (0.22 µm). The membranes were immersed in 5% non‐fat milk at room temperature for 2 h and then incubated with primary antibodies at 4°C for 16–18 h. The primary antibodies were as follows: anti‐TIAR (1:500, Proteintech), anti‐MMP14 (1:1000, Proteintech) and anti‐GAPDH (1:10000, Proteintech). Next, the membranes were incubated with secondary antibodies at room temperature for 2 h, and bands were detected using enhanced chemiluminescence (ECL) reagents from the ECL Detection System. The relative expressions of the proteins were calculated based on the internal reference, GAPDH.
2.5. Cell transfection
Full‐length plasmid TIAR (TIAR[+]), LOXL1‐AS1 (LOXL1‐AS1[+]), miR‐374b‐5p (pre‐miR‐374b‐5p), MMP14 (MMP14[+]) and their negative controls (NC) were constructed by GenePharma (Shanghai, China). The shRNAs against TIAR (TIAR[−]), LOXL1‐AS1 (LOXL1‐AS1[−]), miR‐374b‐5p (anti‐miR‐374b‐5p) and MMP14 (MMP14[−]), and their negative controls were constructed by GeneChem. After cell fusion was approximately 70–80%, the cells were transfected in a 24‐well plate with Lipofectamine 3000. To screen for stably expressing transfected cells, we added G418, puromycin or hygromycin B to the medium. The transfection efficiency was determined using qRT‐PCR and Western blotting (Figure S1). To identify the effects of TIAR on biological behaviour and VM in glioma, we divided the cells into five groups: control, TIAR(−)NC, TIAR(−), TIAR(+)NC and TIAR(+). To investigate the corresponding effects of LOXL1‐AS1, we divided the cells into five groups: control, LOXL1‐AS1(−)NC, LOXL1‐AS1(−), LOXL1‐AS1(+)NC and LOXL1‐AS1(+). To explore the effects of overexpression of TIAR combined with knockdown of LOXL1‐AS1 on malignant cell behaviours and VM, we divided the cells into five groups: control, TIAR(+)NC+LOXL1‐AS1(−)NC, TIAR(+)+LOXL1‐AS1(−)NC, TIAR(+)NC+LOXL1‐AS1(−) and TIAR(+)+LOXL1‐AS1(−). To explore whether LOXL1‐AS1 had a rescue effect on TIAR, we divided the cells into three groups: control, TIAR(+)+LOXL1‐AS1(+)NC and TIAR(+)+LOXL1‐AS1(+). To explore the effects of miR‐374b‐5p on malignancy and VM in glioma, we divided the cells into five groups: control, pre‐NC, pre‐miR‐374b‐5p, anti‐NC and anti‐miR‐374b‐5p. To confirm whether miR‐374b‐5p expression correlates with that of LOXL1‐AS1, we divided the cells into five groups: control, LOXL1‐AS1(−)NC+pre‐NC, LOXL1‐AS1(−)+pre‐miR‐374b‐5p, LOXL1‐AS1(−)NC+anti‐NC and LOXL1‐AS1(−)+anti‐miR‐374b‐5p. To identify the mechanism of MMP14 interaction with miR‐374b‐5p in VM, we divided the cells into five groups: control, pre‐NC+MMP14(−)NC, pre‐miR‐374b‐5p+MMP14(−), pre‐NC+MMP14(+)NC and pre‐miR‐374b‐5p+MMP14(+).
2.6. RNA immunoprecipitation (RIP)
Following the manufacturer's protocol, we performed RIP assays in which the whole‐cell lysate was incubated with magnetic beads from the EZ‐Magna RIP kit (Millipore, Billerica, MA). The beads were conjugated with anti‐Ago 2 antibody or normal mouse IgG. After incubation in proteinase K buffer, the immunoprecipitated RNA was extracted and analysed by qRT‐PCR.
2.7. Fluorescence in situ hybridization (FISH)
After blocking in prehybridization buffer, slides were disposed with PCR‐grade proteinase‐k (Roche Diagnostics, Germany). LOXL1‐AS1 probe (GenePharma), which was constructed to confirm the localization of LOXL1‐AS1 in glioma cells, was added to the hybridization solution. Afterwards, the sections were stained with anti‐digoxin rhodamine conjugate (Exon Biotech Inc.,) at 37°C for 1 h and then DAPI (Beyotime) for 2 min. All images were captured under a fluorescence microscope.
2.8. Dual‐luciferase reporter assays
To investigate the interplay of LOXL1‐AS1 and miR‐374b‐5p, HEK293T cells were co‐transfected with LOXL1‐AS1‐Wt or LOXL1‐AS1‐Mut vector after seeding in a 96‐well plate, and the effect of overexpression of miR‐374b‐5p vs. NC was assessed. To investigate the interplay of miR‐374b‐5p and MMP14, HEK293T cells were co‐transfected with MMP14‐3′UTR‐Wt or MMP14‐3′UTR‐Mut vector, and the overexpression of miR‐374b‐5p vs. NC was assessed. After 48 h, the relative luciferase activity was calculated and analysed using the Dual‐Luciferase Reporter Assay System (Promega).
2.9. Nascent RNA assay
Per the manufacturer's protocol, the expression of nascent LOXL1‐AS1 was examined using the Click‐iT Nascent RNA Capture Kit (Life Technologies Corporation). In general, we used 0.2 mM of ethylene uridine (EU) ribonucleotide homologs to mark the nascent RNA, and the RNA so labelled was released from the magnetic beads and collected. Finally, the nascent RNA expression was determined using qRT‐PCR.
2.10. RNA stability assay
After transfection with TIAR(+) and its NC, HEK293 cells were treated with Actinomycin D (5 mg/ml, Sigma‐Aldrich), and the total RNA was obtained in real time and detected using qRT‐PCR.
2.11. Cell proliferation assay
To assess the cell viability, cells were resuspended and seeded into a 96‐well plate. The Cell Counting Kit‐8 reagent was used per the instructions of the manufacturer. The cell viability was determined using a microplate reader.
2.12. Cell migration and invasion assays
Migration assay: After re‐suspension in a serum‐free medium, approximately 1 × 105 U87 or U251 cells were seeded into a transwell. Simultaneously, the cell‐culture medium was added to the side beneath the transwell chamber. Next, the cells were fixed and stained with 10% Giemsa in phosphate buffer at room temperature overnight. Then, cell numbers were counted in three random fields under a microscope. Modification for invasion assay: We coated 70 μl of Matrigel solution (Corning) with a density of 50 mg/ml on the upper side of the transwell before seeding the cells.
2.13. Tube formation assay
The 3D model tube was formed using a gel. After U87 and U251 cells were successfully transfected, we resuspended the cells and seeded them into a 24‐well plate pre‐coated with Matrigel. Tube formation was then observed and analysed in three random fields under a microscope immediately after incubation at 37°C for nearly 6 h.
2.14. CD34 and PAS dual staining
Tissue specimens were fixed in 4% paraformaldehyde and embedded in paraffin, microtomed, and de‐paraffinized in xylene and graded ethanol solutions. Specimens were then placed in a citrate antigen retrieval solution and heated in a microwave oven at a temperature controlled at close to the boiling point. After subsequent incubation with 3% hydrogen peroxide and goat serum, the specimens were incubated in CD34 primary antibody at a ratio of 1:100 at 4°C overnight. Subsequently, the specimens were incubated with the secondary antibody at room temperature for 20 min and stained with a DAB kit immediately. The PAS reaction was performed using periodic acid and Schiff staining reagents. The specimens were then permanently preserved with neutral resins and stained with haematoxylin. The number of VM tubes was counted and analysed using a normal microscope.
2.15. Xenograft tumour in nude mice
Glioma cells were transfected and screened for stable expression. An in vivo xenograft tumour model was then established in nude mice using stably expressing transfected cells. BALB/C athymic nude mice aged 4 weeks old with a weight of 14–16 g (Beijing HUAFUKANG bioscience) were divided into five groups: control, TIAR(+), LOXL1‐AS1(−), pre‐miR‐374b‐5p and TIAR(+)+LOXL1‐AS1(−)+pre‐miR‐374b‐5p. Each group contained ten mice to ensure experimental accuracy. After transfection and re‐suspension at a density of 2 × 106/ml, 100 ml of cell suspension was injected subcutaneously into each mouse. The volume of tumours was measured and calculated every four days using the following formula: volume (mm3) = length × width2/2. All mice were sacrificed after 44 days. We implanted 1 × 105 cells into the right striatum of nude mice in the orthotopic inoculation experiments. Survival time was analysed using the Kaplan‐Meier survival curve.
Importantly, this research was approved by a panel of experts on laboratory animal care and was conducted following the standards for the care and handling of laboratory animals.
2.16. Statistical analysis
All experimental data shown as mean ± standard deviation were analysed using SPSS 22.0. Statistical significance was set at p < 0.05.
3. RESULTS
3.1. TIAR shows low expression levels in glioma tissues and cells, and its overexpression represses VM by glioma cells
To detect TIAR expression, Western blot analysis was performed. The results demonstrated that TIAR expression was lower in glioma tissues and cells than those in NBTs (Figure 1A) and NHA cells (Figure 1B). To investigate the role of TIAR in glioma, we evaluated the effects of TIAR overexpression and knockdown on cell proliferation, migration, invasion and VM. We observed that MMP14 was downregulated in the TIAR(+) group and upregulated in the TIAR(−) group in comparison with the corresponding NC group (Figure 1C). Cell proliferation, migration, invasion and VM were repressed in the TIAR(+) group but elevated in the TIAR(−) group compared to their NC groups (Figure 1D–F).
FIGURE 1.
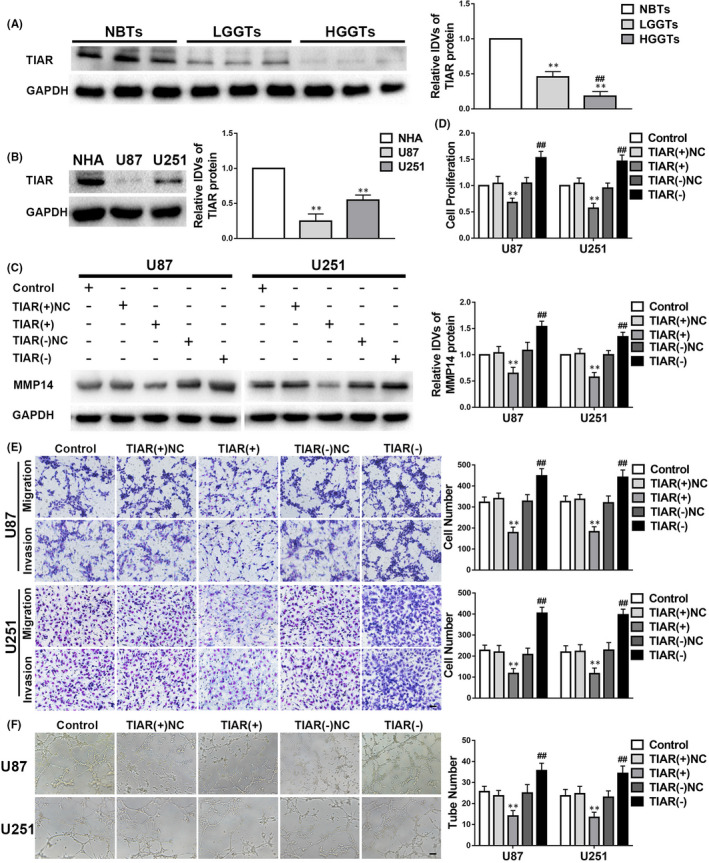
TIA1‐related protein (TIAR) plays a suppressive role in glioma. A–F. The data are displayed as mean ± standard deviation. A. The TIAR expression in glioma tissues is shown. n = 8, normal brain tissues (NBTs); n = 13, low‐grade glioma tissues (LGGTs); n = 18, high‐grade glioma tissues (HGGTs); **p < 0.01 vs. NBTs; ##p < 0.01 vs. LGGTs. B. TIAR expression in glioma cells. n = 5, each group; **p < 0.01 vs. NHA. C. MMP14 expression is regulated by TIAR. n = 5, each group; negative controls (NC); **p < 0.01 vs. TIAR(+)NC; ##p < 0.01 vs. TIAR(−)NC. D–F. Proliferation, migration, invasion, and vasculogenic mimicry (VM) are regulated by TIAR. n = 3, each group; **p < 0.01 vs. TIAR(+)NC; ##p < 0.01 vs. TIAR(−)NC. Scale bars indicate 100 μm in the migration and invasion assays and 200 μm in the tube formation test of VM
3.2. LOXL1‐AS1 is upregulated in glioma tissues and cells, and its knockdown suppresses VM in glioma cells
LOXL1‐AS1 expression was higher in glioma tissues and cells than in NBTs and NHA, and the higher the grade of glioma, the higher the expression of LOXL1‐AS1 (Figure 2A,B). The FISH experiment confirmed that LOXL1‐AS1 was located in the cytoplasm of the NHA and glioma cells (Figure 2D). To explore the mechanism of action of LOXL1‐AS1 in glioma, experiments involving cell proliferation, migration, invasion and VM were conducted shortly after LOXL1‐AS1 was downregulated. We found that the expression of MMP14 in the LOXL1‐AS1(−) group was downregulated compared with that in the LOXL1‐AS1(−)NC group (Figure 2C). Furthermore, as shown in Figure 2E–G, cell proliferation, invasion, migration and VM in the LOXL1‐AS1(−) group were strikingly suppressed in comparison with those in the LOXL1‐AS1(−)NC group.
FIGURE 2.
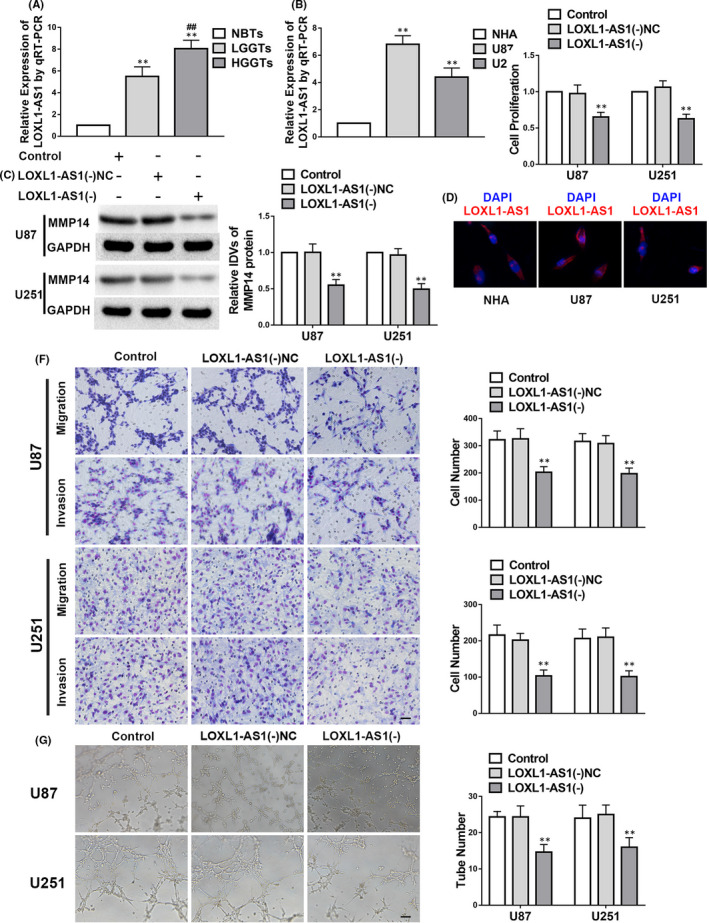
LOXL1‐AS1 functions as an oncogene in glioma. A–G. The data are displayed as mean ± standard deviation. A. LOXL1‐AS1 expression in glioma tissues; n = 8, normal brain tissues (NBTs); n = 13, low‐grade glioma tissues (LGGTs); n = 18, high‐grade glioma tissues (HGGTs); **p < 0.01 vs. NBTs; ##p < 0.01 vs. LGGTs. B. LOXL1‐AS1 expression in glioma cells; n = 5, each group; **p < 0.01 vs. normal human astrocytes. C. Matrix metalloproteinase 14 (MMP14) expression is modulated by LOXL1‐AS1. n = 3, each group; negative controls (NC); **p < 0.01 vs. LOXL1‐AS1(−)NC. D. FISH was performed to examine the location and expression of LOXL1‐AS1 in NHA, U87 and U251. E‐G. Proliferation, migration, invasion and vasculogenic mimicry are regulated by TIAR. n = 3, each group; **p < 0.01 vs. LOXL1‐AS1(−)NC. Scale bars indicate 100 μm in the migration and invasion assays and 200 μm in the tube formation assay
3.3. TIAR reduces LOXL1‐AS1 expression by destabilization, and TIAR overexpression and LOXL1‐AS1 knockdown both significantly reduce VM by glioma cells
Using the Starbase bioinformatics database, we predicted that TIAR binds to LOXL1‐AS1. Results showed that LOXL1‐AS1 was downregulated in the TIAR(+) group but upregulated in the TIAR(−) group compared with the respective NC groups (Figure 3A). To further evaluate the correlation between TIAR and LOXL1‐AS1, TIAR and LOXL1‐AS1 were co‐transfected. As shown in Figure 3F, MMP14 expression was decreased in the TIAR(+)+LOXL1‐AS1(−)NC and TIAR(+)NC+LOXL1‐AS1(−) groups in comparison with that in the TIAR(+)NC+LOXL1‐AS1(−)NC group. Furthermore, we found that MMP14 was significantly downregulated in the TIAR(+)+LOXL1‐AS1(−) group. Figure 3B demonstrates a relative enrichment of LOXL1‐AS1 in the RIP experiment as an elevation in the anti‐TIAR group in comparison with the anti‐IgG group. Moreover, nascent RNA and RNA stability experiments showed that nascent LOXL1‐AS1 expression had no statistical difference among the TIAR(−), TIAR(+), TIAR(−)NC and TIAR(+)NC groups (Figure 3C). The half‐life of LOXL1‐AS1 was shortened in the TIAR(+) group compared with the TIAR(+)NC group (Figure 3D,E). Additionally, cell proliferation, migration, invasion and VM were attenuated in the TIAR(+)+LOXL1‐AS1(−)NC and TIAR(+)NC+LOXL1‐AS1(−) groups and were remarkably reduced in the TIAR(+)+LOXL1‐AS1(−) group in comparison with their respective NC groups (Figure 3G–I).
FIGURE 3.
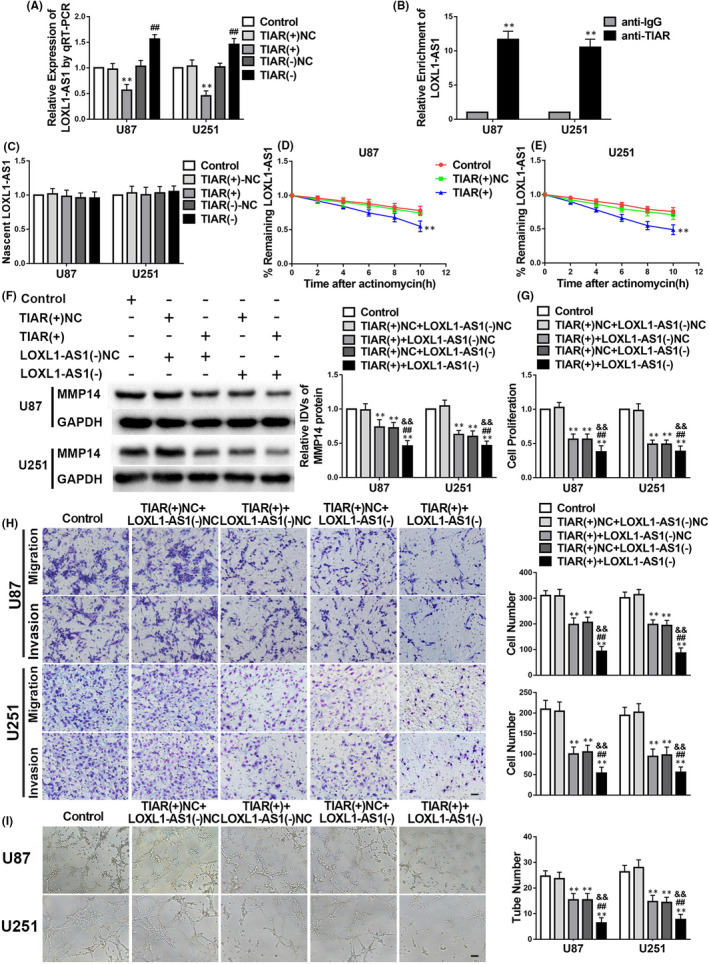
TIA1‐related protein (TIAR) negatively modulates the stability of LOXL1‐AS1 in glioma. A–I. The data are displayed as mean ± standard deviation (n = 3, each group). A. LOXL1‐AS1 expression is regulated by TIAR. negative controls (NC); **p < 0.01 vs. TIAR(+)NC; ##p < 0.01 vs. TIAR(‐)NC. B. LOXL1‐AS1 forms a complex with TIAR. **p < 0.01 vs. anti‐IgG. C–D. The expression levels of the remaining LOXL1‐AS1 in glioma cells are shown a standard time after treatment with actinomycin D. **p < 0.01 vs. TIAR(+)NC. E. Nascent LOXL1‐AS1 expression is shown. F. The protein level of matrix metalloproteinase 14 (MMP14) is regulated by the co‐transfection of TIAR(+) and LOXL1‐AS1(−). negative controls (NC); **p < 0.01 vs. TIAR(+)NC+LOXL1‐AS1(−)NC; ##p < 0.01 vs. TIAR(+)+LOXL1‐AS1(−)NC; &&p < 0.01 vs. TIAR(+)NC+LOXL1‐AS1(−). G–I. The levels of proliferation, migration, invasion and vasculogenic mimicry in glioma cells confirm the effects of co‐transfection of TIAR(+) and LOXL1‐AS1(−). **p < 0.01 vs. TIAR(+)NC+LOXL1‐AS1(−)NC; ##p < 0.01 vs. TIAR(+)+LOXL1‐AS1(−)NC; &&p < 0.01 vs. TIAR(+)NC+LOXL1‐AS1(−). Scale bars indicate 100 μm in the migration and invasion assays and 200 μm in the tube formation assay
3.4. Overexpression of LOXL1‐AS1 reverses the anti‐cancer effects of TIAR overexpression in glioma
The expression of MMP14 in the TIAR(+)+LOXL1‐AS1(+) group was higher than that in the TIAR(+)+LOXL1‐AS1(+)NC group (Figure S2A). Cell proliferation, migration, invasion and VM in the TIAR(+)+LOXL1‐AS1(+) group were also elevated in comparison with those in the TIAR(+)+LOXL1‐AS1(+)NC group (Figure S2B–D).
3.5. miR‐374b‐5p has an anti‐tumour role in glioma, and miR‐374b‐5p overexpression suppresses VM by glioma cells
miR‐374b‐5p was expressed at lower levels in glioma tissues and cells than in NBTs and NHA (Figure 4A,B). To evaluate the function of miR‐374b‐5p in glioma, we evaluated the effects of pre‐miR‐374b‐5p and anti‐miR‐374b‐5p on cell behaviour and VM potential in glioma. As shown in Figure 4C, MMP14 was downregulated in the pre‐miR‐374b‐5p group but upregulated in the anti‐miR‐374b‐5p group compared with their NC groups. Moreover, cell proliferation, migration, invasion and VM were suppressed in the pre‐miR‐374b‐5p group but promoted in the anti‐miR‐374b‐5p group in comparison with their NC groups (Figure 4D–F).
FIGURE 4.
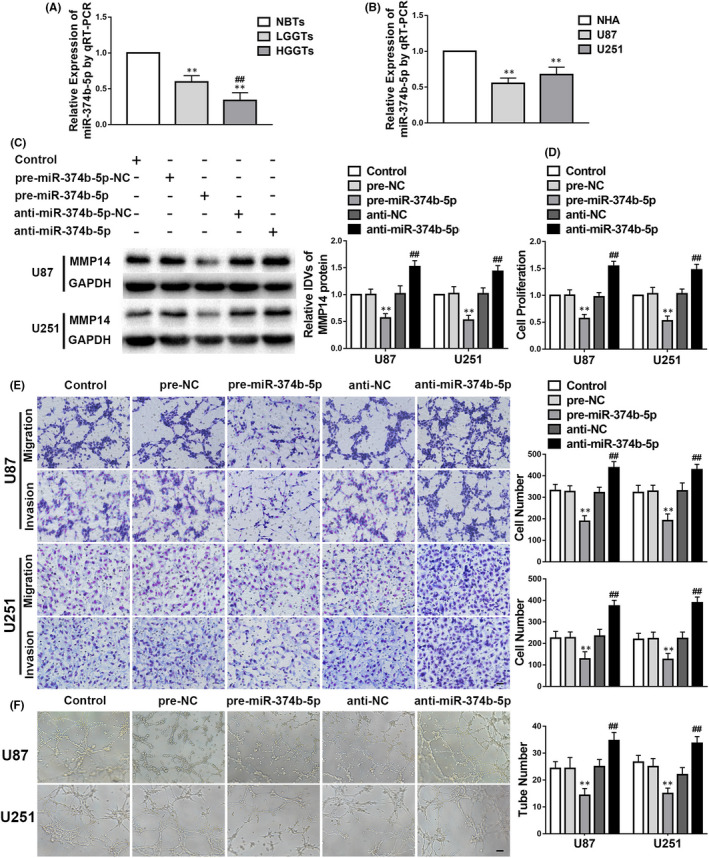
Anti‐tumour effects of miR‐374b‐5p in glioma. A‐F. The data are displayed as mean ± standard deviation. A. miR‐374b‐5p expression in glioma tissues; n = 8, normal brain tissues (NBTs); n = 13, low‐grade glioma tissues (LGGTs); n = 18, high‐grade glioma tissues (HGGTs); **p < 0.01 vs. NBTs; ##p < 0.01 vs. LGGTs. B. miR‐374b‐5p expression in glioma cells; n = 5, each group; **p < 0.01 vs. normal human astrocytes. C. The expression level of matrix metalloproteinase 14 (MMP14) modulated by miR‐374b‐5p; n = 3, each group; negative controls (NC); **p < 0.01 vs. pre‐NC; ##p < 0.01 vs. anti‐NC. D–F. Proliferation, migration, invasion, and vasculogenic mimicry regulated by miR‐374b‐5p; n = 3, each group; **p < 0.01 vs. pre‐NC; ##p < 0.01 vs. anti‐NC. Scale bars indicate 100 μm in the migration and invasion assays and 200 μm in the tube formation assay
3.6. LOXL1‐AS1 downregulates miR‐374b‐5p, and miR‐374b‐5p modulates downregulated LOXL1‐AS1 to affect cell behaviour and VM
Initially, we noticed that miR‐374b‐5p was downregulated in the LOXL1‐AS1(+) group but upregulated in the LOXL1‐AS1(‐) group compared with the corresponding NCs (Figure 5A). Additionally, LOXL1‐AS1 expression was decreased in the pre‐miR‐374b‐5p group but increased in the anti‐miR‐374b‐5p group in comparison with the pre‐NC and anti‐NC groups respectively (Figure 5B). Figure 5C shows that the MMP14 expression was reduced in the LOXL1‐AS1(−)+pre‐miR‐374b‐5p group compared with those of their NCs. Furthermore, the dual‐luciferase reporter assay demonstrated that miR‐374b‐5p binds to LOXL1‐AS1 at its 3′‐UTR (Figure 5D,E). To further investigate this interaction, we evaluated the effects of LOXL1‐AS1 and miR‐374b‐5p on cell proliferation, migration, invasion and VM. As shown in Figure 5F–H, cell proliferation, migration, invasion and VM in glioma (henceforth, ‘malignant cell behaviour’) were greatly inhibited in the LOXL1‐AS1(−)+pre‐miR‐374b‐5p group compared with those of the NCs. We also observed that according to the cell behaviour results, silencing miR‐374b‐5p reversed the tumour‐suppressive function of LOXL1‐AS1(−)+pre‐miR‐374b‐5p.
FIGURE 5.
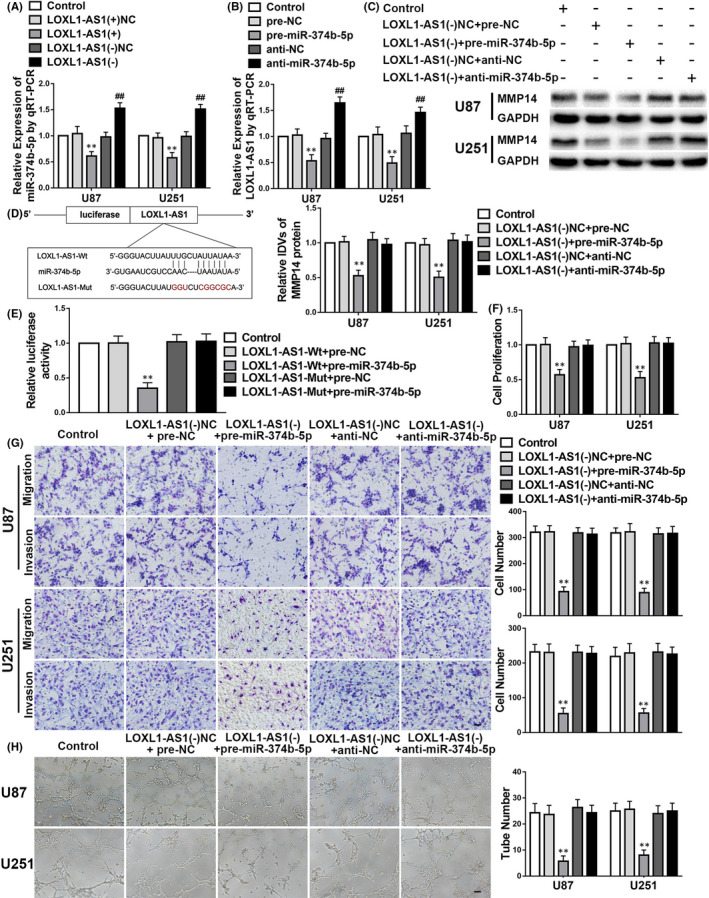
LOXL1‐AS1 knockdown represses malignant cell behaviours and vasculogenic mimicry (VM) in glioma by upregulating miR‐374b‐5p. A–H. The data are displayed as mean ± standard deviation (n = 3, each group). A. miR‐374b‐5p expression is regulated by LOXL1‐AS1. negative controls (NC); **p < 0.01 vs. LOXL1‐AS1(+)NC; ##p < 0.01 vs. LOXL1‐AS1(−)NC. B. LOXL1‐AS1 expression is regulated by miR‐374b‐5p. **p < 0.01 vs. pre‐NC; ##p < 0.01 vs. anti‐NC. C. The sequence mediating binding between LOXL1‐AS1 and miR‐374b‐5p as well as the mutant sequence is shown. D. The relative luciferase activities after the co‐transfection of pre‐miR‐374b‐5p and LOXL1‐AS1‐Mut or LOXL1‐AS1‐Wt are shown. **p < 0.01 vs. LOXL1‐AS1‐Wt+pre‐NC. E. The expression level of matrix metalloproteinase 14 (MMP14) is regulated by co‐transfection of LOXL1‐AS1 and miR‐374b‐5p. **p < 0.01 vs. LOXL1‐AS1(−)NC+pre‐NC. F–H. Cellular proliferation, migration, invasion and VM results confirm the co‐operative action of LOXL1‐AS1 and miR‐374b‐5p transfection in glioma. **p < 0.01 vs. LOXL1‐AS1(−)NC+pre‐NC. Scale bars indicate 100 μm in the migration and invasion assays and 200 μm in the tube formation assay
3.7. MMP14 has an oncogenic role in gliomas and exerts facilitation in VM
As shown in Figure S3A,B, MMP14 was highly expressed in glioma tissues and cells. To figure out its role in gliomas, we assessed the effects of diverse transfections altering MMP14 expressions on the malignant cell behaviour. The malignant cell behaviour was greatly inhibited in the MMP14(‐) group but promoted in the MMP14 (+) group, in comparison with that of their corresponding NCs (Figure S3C–E).
3.8. miR‐374b‐5p targets MMP14 by binding to its 3′UTR; miR‐374b‐5p and MMP14 both regulate malignant cell behaviour
The dual‐luciferase reporter assay proved that miR‐374b‐5p binds to MMP14 at its 3′‐UTR (Figure 6A,B). To explore the role of this interaction, we assessed the effects of diverse transfections altering the expressions of miR‐374b‐5p and MMP14 on malignant cell behaviour. Malignant cell behaviour was greatly inhibited in the pre‐miR‐374b‐5p+MMP14(−) group compared to that in their corresponding NCs (Figure 6C–E). Moreover, MMP14 overexpression reversed this effect (Figure 6C–E).
FIGURE 6.
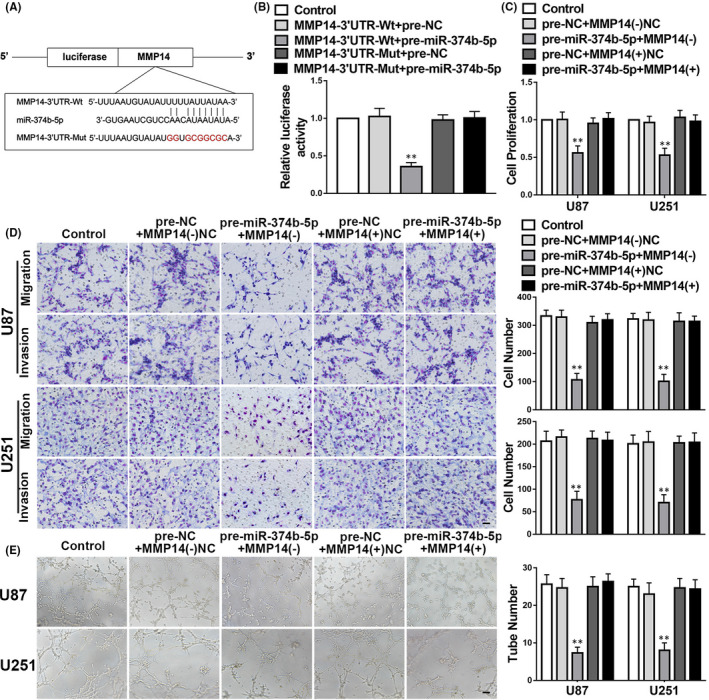
miR‐374b‐5p overexpression represses malignant cellular behaviours and vasculogenic mimicry (VM) in gliomas by targeting MMP14. A. The potential binding sequence for Matrix metalloproteinase 14 (MMP14) and miR‐374b‐5p and a mutant sequence is shown. B–E. The data are displayed as mean ± standard deviation (n = 3, each group). B. The relative luciferase activities for the co‐transfection of pre‐miR‐374b‐5p and either MMP14‐Mut or MMP14‐Wt are shown. negative controls (NC); **p < 0.01 vs. MMP14‐Wt+pre‐NC. C–E. The cellular proliferation, migration, invasion and VM results confirm the effects of co‐transfection of MMP14 and miR‐374b‐5p in gliomas. **p < 0.01 vs. MMP14(−)NC+pre‐NC. Scale bars indicate 100 μm in the migration and invasion assays and 200 μm in the tube formation assay
3.9. Overexpression of TIAR combined with LOXL1‐AS1 knockdown and overexpression of miR‐374b‐5p inhibits the growth and VM propensity of xenograft tumours in nude mice and prolongs survival
A nude mouse xenograft model was established to investigate the functions of TIAR, LOXL1‐AS1 and miR‐374b‐5p in glioma in vivo. The results demonstrated that the volume of xenograft tumours was smaller in the TIAR(+), LOXL1‐AS1(−) and pre‐miR‐374b‐5p groups than that in their NCs, and the smallest in the TIAR(+)+LOXL1‐AS1(−)+pre‐miR‐374b‐5p group (Figure 7A,B). The survival time was prolonged in the TIAR(+), LOXL1‐AS1(−) and pre‐miR‐374b‐5p groups compared to that of their NCs (Figure 7C) and was longest in the TIAR(+)+LOXL1‐AS1(−)+pre‐miR‐374b‐5p group. Finally, the number of VM tubes seen in vivo was lower in the TIAR(+), LOXL1‐AS1(−) and pre‐miR‐374b‐5p groups than that in their NCs and the least in the TIAR(+)+LOXL1‐AS1(−)+pre‐miR‐374b‐5p group (Figure 7D).
FIGURE 7.
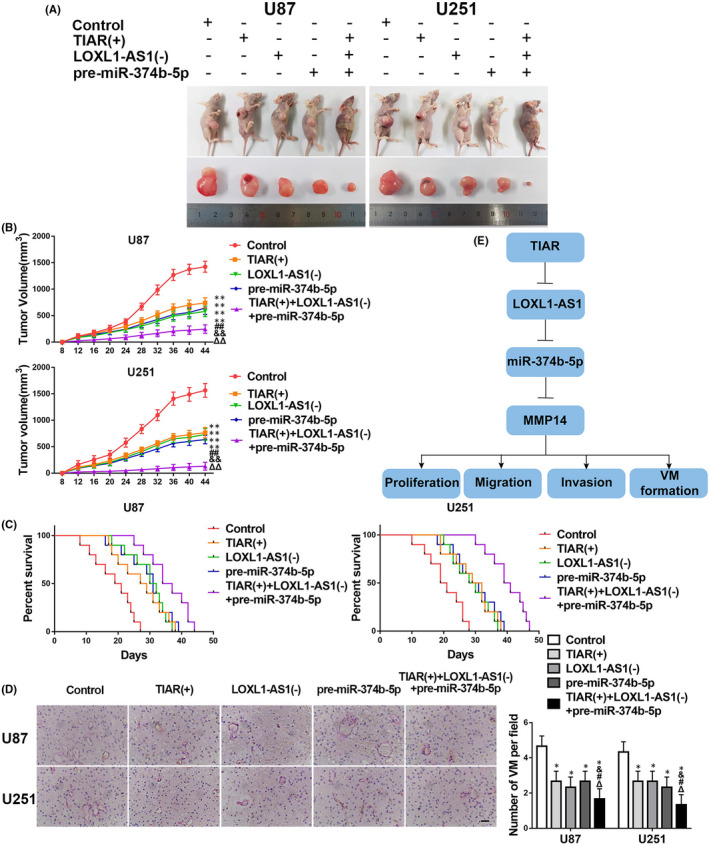
In vivo study. A. The tumours in vivo and the corresponding samples following the subcutaneous implantation of stably expressing cells into nude mice are shown. B. The tumour volumes are shown. The data are displayed as mean ± standard deviation (SD) (n = 10, each group). **p < 0.01 vs. control group; ##p < 0.01 vs. TIAR(+) group; &&p < 0.01 vs. LOXL1‐AS1(−) group; ΔΔp < 0.01 vs. pre‐miR‐374b‐5p group. C. The survival curves of the nude mice groups are shown. p < 0.05 (TIAR(+), LOXL1‐AS1(−) and pre‐miR‐374b‐5p vs. control). D. VM in xenograft tumours by CD34‐PAS staining. The data are displayed as mean ± SD (n = 5, each group). *p < 0.05 vs. control group; #p < 0.05 vs. TIAR(+) group; &p < 0.05 vs. LOXL1‐AS1(−) group; Δp < 0.05 vs. pre‐miR‐374b‐5p group. Scale bars indicate 200 μm. E. A schematic of the TIA1‐related protein (TIAR)/LOXL1‐AS1/miR‐374b‐5p mechanism
4. DISCUSSION
In this study, we showed that TIAR exerted anti‐tumour effects, while LOXL1‐AS1 acted as an oncogene in glioma. TIAR could combine with LOXL1‐AS1 and reduce its expression by lowering its stability. Moreover, TIAR overexpression and LOXL1‐AS1 knockdown attenuated cell viability, migration, invasion and VM ability. However, LOXL1‐AS1 overexpression reversed the anti‐cancer effects of TIAR overexpression in glioma cells. Our observations also showed that miR‐374b‐5p functions as a tumour suppressor in gliomas. Subsequently, we proved that LOXL1‐AS1 was an RNA sponge for miR‐374b‐5p and downregulated its expression. Additionally, MMP14 acts as an oncogene in gliomas, while miR‐374b‐5p could mediate MMP14 expression by binding to their 3′UTRs, thus attenuating the malignant cell behaviours and VM in glioma. Finally, the concerted action of overexpressed TIAR, silenced LOXL1‐AS1 and overexpressed miR‐374b‐5p repressed tumour growth as well as VM in vivo and prolonged the survival of nude mice.
RBPs play key functions in tumorigenesis and development at splicing, transcriptional, translational, intracellular‐transport and modification levels. The RBP NONO plays an oncogenic role in breast cancer and modifies SKP2 and E2F8 in the post‐transcriptional phase. 27 SORBS2 enhanced the stability of WFDC1 and IL‐17D and inhibited the invasion in ovarian cancer. 28 A recent study suggested that the lncRNA MT1JP communicating with TIAR post‐transcriptionally regulates P53 in tumours. 29 In our study, TIAR was expressed at low levels in glioma. TIAR overexpression remarkably inhibited proliferation, metastasis and tube formation, while its knockdown tended to act in the opposite direction, confirming its tumour suppression role in glioma.
Malfunctions of lncRNAs play a unique role in oncogenesis and progression. A recent study identified the promotion of lnc_000231 in cervical cancer, which acts by interacting with miR‐497‐5p. 30 The lncRNA ATB binds to EZH2 and downregulates the expression of DAB2IP, CDH1, LATS2, FOXC1 and CDX1, thus facilitating the progression of ovarian cancer. 31 Furthermore, our study showed that LOXL1‐AS1 is highly expressed in glioma tissues and cells, and its downregulation repressed the proliferation, metastasis and VM ability of these cells. These results confirmed that LOXL1‐AS1 has an aggressive role in glioma. Silencing LOXL1‐AS1 suppresses cell proliferation in glioblastoma, 32 which is consistent with our findings. Moreover, LOXL1‐AS1 enhances the proliferation and invasion in medulloblastoma 33 and negatively modulates miR‐3128, resulting in attenuation of the malignancy of H1299 and A549 lung cancer cells. 34 However, the relationship between TIAR and LOXL1‐AS1 in regulating VM formation in glioma has not been explored.
Several studies have reported that RBPs can positively or negatively affect RNA stability, resulting in tumour growth. For example, LARP1 enhances the stability of BCL2 but attenuates the stability of BIK, leading to the malignant progression of ovarian cancer. 35 Linc‐00313, which is stabilized by UPF1, regulates miR‐342‐3p and miR‐485‐5p, eventually accelerating the progression of glioblastoma. 36 In colorectal cancer, MBNL1 destabilizes Snail and inhibits the epithelial‑to‑mesenchymal transition and metastasis of tumour cells. 37 In our study, LOXL1‐AS1 expression was reduced in TIAR‐overexpressing glioma cells and increased in TIAR‐knockdown glioma cells. Moreover, the results showed that TIAR communicated with LOXL1‐AS1 but had no effect on levels of the nascent transcript. It therefore decreased LOXL1‐AS1 expression by reducing its half‐life. Co‐transfection with TIAR overexpression and LOXL1‐AS1 knockdown plasmids greatly mediated the proliferation, migration, invasion and VM of glioma cells. Our results revealed that TIAR’s weakening of the stability of LOXL1‐AS1 attenuated the progression of glioma. Moreover, overexpression of LOXL1‐AS1 reversed the anti‐tumour effects of TIAR overexpression in glioma cells. The function of LOXL1‐AS1 in promoting adverse biological behaviours and VM in glioma has not been previously established.
Growing evidence has focused on the role of the relationship between lncRNAs and miRNAs in the mechanism of tumorigenesis. For example, LINC‐PINT modified miR‐767‐5p/TET2 and inhibited malignant behaviour in thyroid cancer. 38 lnc‐NEAT1 acts as an oncogene to sponge miR‐486‐5p, and its suppression downregulates the malignant progression of colorectal cancer. 39 The lncRNA EBLN3P, functioning as a competitive sponge of miRNA‐144‐3p, positively modulates DOCK4 in the ceRNA pathway and facilitates adverse processes in liver cancer. 40 To confirm the function of LOXL1‐AS1 in glioma, we used Starbase and predicted miR‐374b‐5p as a target of LOXL1‐AS1. A dual‐luciferase reporter assay was performed to assess whether LOXL1‐AS1 binds to miR‐374b‐5p. LOXL1‐AS1 upregulation suppressed miR‐374b‐5p expression, while miR‐374b‐5p overexpression reduced LOXL1‐AS1 expression. Moreover, miR‐374b‐5p downregulation reduced the anti‐tumour effects of sh‐LOXL1‐AS1 in glioma. These results indicate that LOXL1‐AS1 exerts sponge‐like effect on miR‐374b‐5p, thereby regulating malignant cellular behaviours and VM in glioma. Consistent with our reports, LOXL1‐AS1 sponges miR‐541‐3p by interacting with CCND1, thereby driving cell cycle progression and proliferation in prostate cancer. 41 LOXL1‐AS1 facilitates the adverse processes of gastric cancer by modulating miR‐142‐5p in a sponge way. 42 Additionally, LOXL1‐AS1 could regulate miR‐324‐3p like a sponge and accelerate the pernicious processes of cholangiocarcinoma and non‐small‐cell lung cancer. 43 , 44
Herein, we established an implanted tumour model in nude mice and observed the minimum tumour volume and longest survival in the TIAR(+)+LOXL1‐AS1(−)+pre‐miR‐374b‐5p group. Furthermore, dual staining for CD34 and PAS demonstrated that VM tubes were the fewest in the co‐transfected group. To date, it has been established that TIAR combined with LOXL1‐AS1 can regulate miR‐374b‐5p; however, the mechanism by which miR‐374b‐5p regulates VM formation in glioma has not been proven.
miRNA dysfunction is found in most tumour cellular processes. For instance, miR‐3666 targets STAT3 and modulates the activity of AK4, exhibiting a suppressive role in ovarian cancer. 45 miRNA‐506‐3p targets EZH2 and negatively modulates the expression of β‐catenin in serous ovarian cancer. 46 In this study, miR‐374b‐5p was downregulated in glioma, which revealed its function as a tumour suppressor. Similarly, miR‐374b‐5p targets FOXP1 and exerts a protective role in non–small‐cell lung cancer and ovarian cancer. 47 , 48 Additionally, both miR‐374b‐5p and miR‐454‐3p exert inhibitory effects by regulating ZEB2 in bladder cancer. 49 Moreover, miR‐374b targets FOXM1, and its overexpression mediates adverse effects in cervical cancer. 50 We also proved that MMP14 promotes malignant cell behaviours in gliomas. Likewise, MMP14 is highly expressed in gliomas where it acts as a mediator of migration. 51 We also noticed that miR‐374b‐5p restrained MMP14 by binding to its 3′UTRs according to the dual‐luciferase reporter assay and Western blot, leading to the inhibition of VM by glioma cells. Similarly, MMP2 and MMP9 function as the key mediator of VM in glioma, while the suppression of Tenascin‐c attenuates AKT phosphorylation, and downregulates MMP2 and MMP9 expression, thus repressing VM in gliomas. 52 Additionally, the upregulation of MMP14 reduced the inhibitory effects of overexpressed miR‐374b‐5p in glioma cells.
Overall, our study shows for the first time that TIAR plays a tumour‐suppressive role, while LOXL1‐AS1 plays an aggressive role in glioma. TIAR downregulates LOXL1‐AS1 by reducing its stability. LOXL1‐AS1 overexpression reversed the anti‐tumour effects of TIAR overexpression in glioma cells. Moreover, LOXL1‐AS1 knockdown upregulated miR‐374b‐5p, leading to the downregulation of MMP14, eventually suppressing malignant cell behaviour and VM in glioma cells. Our study shows that the TIAR/LOXL1‐AS1/miR‐374b‐5p/MMP14 axis has significant effects in regulating VM in glioma, which could help reveal novel targets for glioma therapy.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Bolong Yi: Data curation (equal); Funding acquisition (equal); Investigation (lead); Writing – original draft (lead); Writing – review & editing (equal). Hao Li: Funding acquisition (equal); Investigation (equal); Methodology (equal); Software (equal). Heng Cai: Data curation (equal); Methodology (equal). Xin Lou: Formal analysis (equal); Investigation (equal); Software (equal); Writing – review & editing (equal). Mingjun Yu: Formal analysis (equal); Software (equal). Zhen Li: Data curation (equal); Funding acquisition (lead); Supervision (lead); Writing – review & editing (equal).
ETHICAL APPROVAL
Written informed consent was signed voluntarily by all patients. This research is supported by the Shengjing Hospital Ethical Committee (approval ID: 2020PS012K) and the panel of experts on Laboratory Animal Care of the Shengjing Hospital (approval ID: 2020PS015K).
Supporting information
Fig S1
Fig S2
Fig S3
Table S1
ACKNOWLEDGEMENTS
Our research is supported by Liaoning Science and Technology Plan Project (ME86); outstanding scientific fund of Shengjing hospital (M0214, M0167).
Yi B, Li H, Cai H, Lou X, Yu M, Li Z. LOXL1‐AS1 communicating with TIAR modulates vasculogenic mimicry in glioma via regulation of the miR‐374b‐5p/MMP14 axis. J Cell Mol Med.2022;26:475–490. doi: 10.1111/jcmm.17106
DATA AVAILABILITY STATEMENT
We confirm that we will share the data underlying the findings reported in this manuscript and allow researchers to verify the results presented, replicate the analysis, and conduct secondary analyses.
REFERENCES
- 1. Mu Y, Tang Q, Feng H, Zhu L, Wang Y. lncRNA KTN1‐AS1 promotes glioma cell proliferation and invasion by negatively regulating miR‐505‐3p. Oncol Rep. 2020;44(6):2645‐2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cai H, Yu Y, Ni X, et al. LncRNA LINC00998 inhibits the malignant glioma phenotype via the CBX3‐mediated c‐Met/Akt/mTOR axis. Cell Death Dis. 2020;11(12):1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Q, Yang L, Guan G, Cheng P, Cheng W, Wu A. LOXL2 upregulation in gliomas drives tumorigenicity by activating autophagy to promote TMZ resistance and trigger EMT. Front Oncol. 2020;10:569584. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4. Fan W, Song Y, Ren Z, et al. Glioma cells are resistant to inflammation‐induced alterations of mitochondrial dynamics. Int J Oncol. 2020;57(6):1293‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mei X, Chen YS, Chen FR, Xi SY, Chen ZP. Glioblastoma stem cell differentiation into endothelial cells evidenced through live‐cell imaging. Neuro‐oncology. 2017;19(8):1109‐1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang D, Ruan X, Liu X, et al. SUMOylation of PUM2 promotes the vasculogenic mimicry of glioma cells via regulating CEBPD. Clin Transl Med. 2020;10(5):e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qiao K, Liu Y, Xu Z, et al. RNA m6A methylation promotes the formation of vasculogenic mimicry in hepatocellular carcinoma via Hippo pathway. Angiogenesis. 2020;24(1):83‐96. [DOI] [PubMed] [Google Scholar]
- 8. Zhao J, Wu J, Qin Y, Zhang W, Huang G, Qin L. LncRNA PVT1 induces aggressive vasculogenic mimicry formation through activating the STAT3/Slug axis and epithelial‐to‐mesenchymal transition in gastric cancer. Cell Oncol (Dordr). 2020;43(5):863‐876. [DOI] [PubMed] [Google Scholar]
- 9. Zhao B, Wu M, Hu Z, et al. Thrombin is a therapeutic target for non‐small‐cell lung cancer to inhibit vasculogenic mimicry formation. Signal Transduct Target Ther. 2020;5(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zong S, Tang Y, Li W, et al. αA Chinese herbal formula suppresses colorectal cancer migration and vasculogenic mimicry through ROS/HIF‐1/MMP2 pathway in hypoxic microenvironment. Front Pharmacol. 2020;11:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pan M, Wang H, Ansari KH, Li XP, Sun W, Fan YZ. Gallbladder cancer‐associated fibroblasts promote vasculogenic mimicry formation and tumor growth in gallbladder cancer via upregulating the expression of NOX4, a poor prognosis factor, through IL‐6‐JAK‐STAT3 signal pathway. J Experim Clin Cancer Res. 2020;39(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu X, Zhang QP, Mu YG, et al. Clinical significance of vasculogenic mimicry in human gliomas. J Neurooncol. 2011;105(2):173‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gebauer F, Schwarzl T, Valcárcel J, Hentze MW. RNA‐binding proteins in human genetic disease. Nat Rev Genet. 2020;22(3):185‐198. [DOI] [PubMed] [Google Scholar]
- 14. Sánchez‐Jiménez C, Ludeña MD, Izquierdo JM. T‐cell intracellular antigens function as tumor suppressor genes. Cell Death Dis. 2015;6(3):e1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weeks A, Agnihotri S, Lymer J, et al. Epithelial Cell transforming 2 and aurora kinase B modulate formation of stress granule‐containing transcripts from diverse cellular pathways in astrocytoma cells. Am J Pathol. 2016;186(6):1674‐1687. [DOI] [PubMed] [Google Scholar]
- 16. Dong HT, Liu Q, Zhao T, et al. Long non‐coding RNA LOXL1‐AS1 drives breast cancer invasion and metastasis by antagonizing miR‐708‐5p expression and activity. Mol Ther Nucleic Acids. 2020;19:696‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bai T, Liu Y, Li B. LncRNA LOXL1‐AS1/miR‐let‐7a‐5p/EGFR‐related pathway regulates the doxorubicin resistance of prostate cancer DU‐145 cells. IUBMB Life. 2019;71(10):1537‐1551. [DOI] [PubMed] [Google Scholar]
- 18. He G, Yao W, Li L, Wu Y, Feng G, Chen L. LOXL1‐AS1 contributes to the proliferation and migration of laryngocarcinoma cells through miR‐589‐5p/TRAF6 axis. Cancer Cell Int. 2020;20:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xue F, Xu YH, Shen CC, Qin ZL, Zhou HB. Non‐coding RNA LOXL1‐AS1 exhibits oncogenic activity in ovarian cancer via regulation of miR‐18b‐5p/VMA21 axis. Biomed Pharmacother. 2020;125:109568. [DOI] [PubMed] [Google Scholar]
- 20. Wu X, Cui F, Chen Y, Zhu Y, Liu F. Long non‐coding RNA LOXL1‐AS1 enhances colorectal cancer proliferation, migration and invasion through miR‐708‐5p/CD44‐EGFR axis. Onco Targets Ther. 2020;13:7615‐7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cui Y, Liang S, Zhang S, et al. ABCA8 is regulated by miR‐374b‐5p and inhibits proliferation and metastasis of hepatocellular carcinoma through the ERK/ZEB1 pathway. J Exp Clin Cancer Res. 2020;39(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun D, Wang X, Sui G, Chen S, Yu M, Zhang P. Downregulation of miR‐374b‐5p promotes chemotherapeutic resistance in pancreatic cancer by upregulating multiple anti‐apoptotic proteins. Int J Oncol. 2018;52(5):1491‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li GC, Cao XY, Li YN, et al. MicroRNA‐374b inhibits cervical cancer cell proliferation and induces apoptosis through the p38/ERK signaling pathway by binding to JAM‐2. J Cell Physiol. 2018;233(9):7379‐7390. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Z, Nong L, Chen M, et al. Baicalein suppresses vasculogenic mimicry through inhibiting RhoA/ROCK expression in lung cancer A549 cell line. Acta Biochim Biophys Sin (Shanghai). 2020;52(9):1007‐1015. [DOI] [PubMed] [Google Scholar]
- 25. Cheng R, Wang B, Cai XR, et al. CD276 promotes vasculogenic mimicry formation in hepatocellular carcinoma via the PI3K/AKT/MMPs pathway. Onco Targets Ther. 2020;13:11485‐11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. You X, Wu J, Wang Y, et al. Galectin‐1 promotes vasculogenic mimicry in gastric adenocarcinoma via the Hedgehog/GLI signaling pathway. Aging (Albany NY). 2020;12(21):21837‐21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iino K, Mitobe Y, Ikeda K, et al. RNA‐binding protein NONO promotes breast cancer proliferation by post‐transcriptional regulation of SKP2 and E2F8. Cancer Sci. 2020;111(1):148‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao L, Wang W, Huang S, et al. The RNA binding protein SORBS2 suppresses metastatic colonization of ovarian cancer by stabilizing tumor‐suppressive immunomodulatory transcripts. Genome Biol. 2018;19(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu L, Yue H, Liu Q, et al. LncRNA MT1JP functions as a tumor suppressor by interacting with TIAR to modulate the p53 pathway. Oncotarget. 2016;7(13):15787‐15800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Li X, Zhang J, Mao L, et al. E6 hijacks KDM5C/lnc_000231/miR‐497‐5p/CCNE1 axis to promote cervical cancer progression. J Cell Mol Med. 2020;24(19):11422‐11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen X, An N. Long noncoding RNA ATB promotes ovarian cancer tumorigenesis by mediating histone H3 lysine 27 trimethylation through binding to EZH2. J Cell Mol Med. 2020;25(1):37‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang H, Li L, Yin L. Silencing LncRNA LOXL1‐AS1 attenuates mesenchymal characteristics of glioblastoma via NF‐κB pathway. Biochem Biophys Res Commun. 2018;500(2):518‐524. [DOI] [PubMed] [Google Scholar]
- 33. Gao R, Zhang R, Zhang C, Liang Y, Tang W. LncRNA LOXL1‐AS1 promotes the proliferation and metastasis of medulloblastoma by activating the PI3K/AKT pathway. Anal Cell Pathol (Amst). 2018;2018:9275685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao L, Zhang X, Guo H, Liu M, Wang L. LOXL1‐AS1 contributes to non‐small cell lung cancer progression by regulating miR‐3128/RHOXF2 axis. Onco Targets Ther. 2020;13:6063‐6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hopkins T, Mura M, Al‐Ashtal HA, et al. The RNA‐binding protein LARP1 is a post‐transcriptional regulator of survival and tumorigenesis in ovarian cancer. Nucleic Acids Res. 2016;44(3):1227‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shao L, He Q, Liu Y, et al. UPF1 regulates the malignant biological behaviors of glioblastoma cells via enhancing the stability of Linc‐00313. Cell Death Dis. 2019;10(9):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang L, Zhao P, Kong D. Muscleblind‐like 1 destabilizes Snail mRNA and suppresses the metastasis of colorectal cancer cells via the Snail/E‐cadherin axis. Int J Oncol. 2019;54(3):955‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jia M, Li Z, Pan M, Tao M, Wang J, Lu X. LINC‐PINT suppresses the aggressiveness of thyroid cancer by downregulating miR‐767‐5p to Induce TET2 expression. Mol Ther Nucleic Acids. 2020;22:319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Z, Gu Y, Cheng X, et al. Upregulation lnc‐NEAT1 contributes to colorectal cancer progression through sponging miR‐486‐5p and activating NR4A1/Wnt/β‐catenin pathway. Cancer Biomark. 2021;30(3):309‐319. [DOI] [PubMed] [Google Scholar]
- 40. Li H, Wang M, Zhou H, Lu S, Zhang B. EBLN3PLong noncoding RNA promotes the progression of liver cancer via alteration of microRNA‐144‐3p/DOCK4 signal. Cancer Manag Res. 2020;12:9339‐9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Long B, Li N, Xu XX, et al. Long noncoding RNA LOXL1‐AS1 regulates prostate cancer cell proliferation and cell cycle progression through miR‐541‐3p and CCND1. Biochem Biophys Res Commun. 2018;505(2):561‐568. [DOI] [PubMed] [Google Scholar]
- 42. Li M, Cai O, Tan S. LOXL1‐AS1 drives the progression of gastric cancer via regulating miR‐142‐5p/PIK3CA axis. Onco Targets Ther. 2019;12:11345‐11357. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Zhang B, Zhou M, Zou L, et al. Long non‐coding RNA LOXL1‐AS1 acts as a ceRNA for miR‐324‐3p to contribute to cholangiocarcinoma progression via modulation of ATP‐binding cassette transporter A1. Biochem Biophys Res Commun. 2019;513(4):827‐833. [DOI] [PubMed] [Google Scholar]
- 44. Xie N, Fei X, Liu S, Liao J, Li Y. LncRNA LOXL1‐AS1 promotes invasion and proliferation of non‐small‐cell lung cancer through targeting miR‐324‐3p. Am J Transl Res. 2019;11(10):6403‐6412. [PMC free article] [PubMed] [Google Scholar]
- 45. Tan H, Wu C, Huang B, Jin L, Jiang X. MiR‐3666 serves as a tumor suppressor in ovarian carcinoma by down‐regulating AK4 via targeting STAT3. Cancer Biomark. 2021;30(4):355‐363. [DOI] [PubMed] [Google Scholar]
- 46. Sun Y, Wu J, Dong X, Zhang J, Meng C, Liu G. MicroRNA‐506‐3p increases the response to PARP inhibitors and cisplatin by targeting EZH2/β‐catenin in serous ovarian cancers. Transl Oncol. 2020;14(2):100987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li J, Zhang X, Tang J, Gong C. MicroRNA‐374b‐5p functions as a tumor suppressor in non‐small cell lung cancer by targeting FOXP1 and Predicts prognosis of cancer patients. Onco Targets Ther. 2020;13:4229‐4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li H, Liang J, Qin F, Zhai Y. MiR‐374b‐5p‐FOXP1 feedback loop regulates cell migration, epithelial‐mesenchymal transition and chemosensitivity in ovarian cancer. Biochem Biophys Res Commun. 2018;505(2):554‐560. [DOI] [PubMed] [Google Scholar]
- 49. Wang S, Zhang G, Zheng W, et al. MiR‐454‐3p and miR‐374b‐5p suppress migration and invasion of bladder cancer cells through targetting ZEB2. Biosci Rep. 2018;38(6):BSR20181436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xia N, Tan WF, Peng QZ, Cai HN. MiR‐374b reduces cell proliferation and cell invasion of cervical cancer through regulating FOXM1. Eur Rev Med Pharmacol Sci. 2019;23(2):513‐521. [DOI] [PubMed] [Google Scholar]
- 51. Liu K, Liu J, Bo QF. MFI2‐AS1 regulates the aggressive phenotypes in glioma by modulating MMP14 via a positive feedback loop. Eur Rev Med Pharmacol Sci. 2019;23(13):5884‐5895. [DOI] [PubMed] [Google Scholar]
- 52. Cai H, Wang J, Xi SY, et al. Tenascin‐cmediated vasculogenic mimicry formation via regulation of MMP2/MMP9 in glioma. Cell Death Dis. 2019;10(12):879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Table S1
Data Availability Statement
We confirm that we will share the data underlying the findings reported in this manuscript and allow researchers to verify the results presented, replicate the analysis, and conduct secondary analyses.


