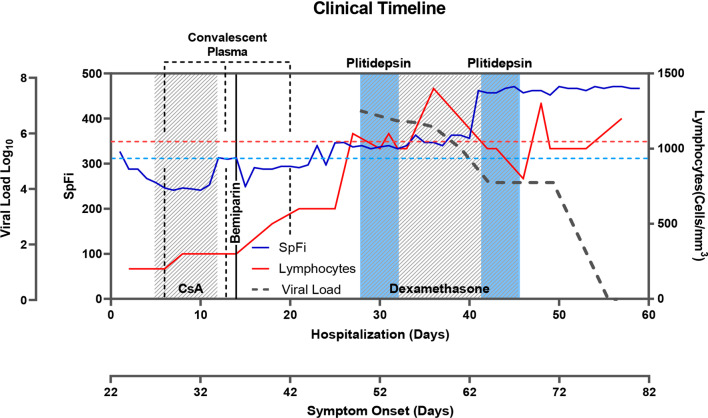
Fig. 1.
Timeline of main laboratory and microbiological parameters during and after plitidepsin therapy. Two timepoints have been taken as reference: the number of days from SARS-CoV-2 symptom onset, and the number of days since hospital admission. Plitidepsin was administered on days 49–51 and 65–67 after symptom onset. Parameters shown in the clinical course are as follows: A Quantitative viral load (log10 copies/ml) using nasopharyngeal swabs samples (grey-dotted line). B SpFI (AU) blue line. C Lymphocyte total count (cells/mm3) (red line). AU arbitrary units, CsA cyclosporine A, SpFi ratio of oxygen saturation in blood (SpO2)/fraction of inspired oxygen (FiO2) at or below 300 AU