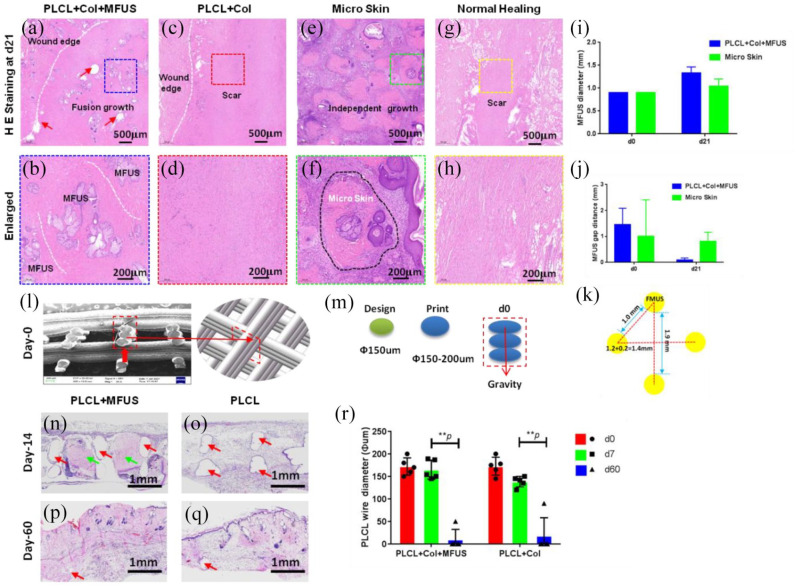
Figure 6.
H& E staining on the healed rat dorsal skin tissue sections at day-21 post-surgery with four different treatments and the degradation of PLCL scaffold in vivo. (a and b) The wound treated with tissue engineering skin (PLCL + Col + MFUS) healed well and formed functional skin with implanted rat tail MFUS (blue dash box areas). (c and d) The wound treated with 3D-printed PLCL scaffold and collagen gel (PLCL + Col) healed completely, but no functional skin formed in the wound area (red dash box areas). (e and f) The wound treated with micro skin islands healed much better than the normal healing wounds and PLCL + Col treated wounds, but some unhealed areas were still found due to the MFUS lost. The green dash box areas and black dash line areas showed the MFUS survival. (g and h) The wound without treatment (Normal healing) showed many loose collagen fibers in the wound area, indicating the healing is poor and some scar tissues have formed (yellow dash box areas). (i) Semi-quantification of MFUS diameter in the wound areas indicated that the MFUSs have grown up in both treated wound areas, but MFUS grew faster in the wounds treated with tissue engineered skin (PLCL + Col + MFUS) than the wounds treated with micro skin only (micro skin). (j) The gap distance between two MFUSs was much smaller in the wound areas treated with tissue engineered skin (PLCL + Col + MFUS) than that of the wounds treated with micro skin islands (Micro skin). (k) A drawn picture showed the distance between two MFUSs in tissue engineering skin at day-0. White dash lines in (a–h) showed the wound edges, and red arrows in (a) indicated the PLCL scaffold. (l) SEM image of the cross-section of the 3D-printed PLCL scaffold shows the structure and size of the wall of PLCL scaffold at day-0 post-surgery (red box area). (m) Drawn pictures showed the diameter of the designed and printed fiber in 3D-printed PLCL scaffold. (n) H&E staining on the cross section of wounded rat skin treated with tissue engineering skin (PLCL + Col + MFUS) at day-14 post-surgery showed the MFUSs (green arrows) have grown up in the PLCL scaffold. The gap areas (red arrows) showed the scaffold has degraded. (o) H&E staining on the cross section of wounded rat skin treated with 3D-printed scaffold and collagen gel (PLCL + Col) at day-14 post-surgery showed the scaffold has degraded and left the empty gap areas without cells (red arrows). (p) H&E staining on the cross section of wounded rat skin treated with tissue engineering skin (PLCL + Col + MFUS) at day-60 post-surgery showed the PLCL scaffold has degraded completely and the most empty areas have been filled with cells. The empty areas were much smaller compared to the scaffold at day-14 (red arrow). (q) H&E staining on the cross section of wounded rat skin treated with 3D-printed scaffold and collagen gel (PLCL + Col) at day-60 post-surgery showed the scaffold has degraded completely, and the left empty areas were much smaller compared to the scaffold at day-14 (red arrow). (r) Semi-quantification of the H&E staining showed that the wire diameter of the scaffold decreased in vivo in a time dependent manner.