Fig. 3.
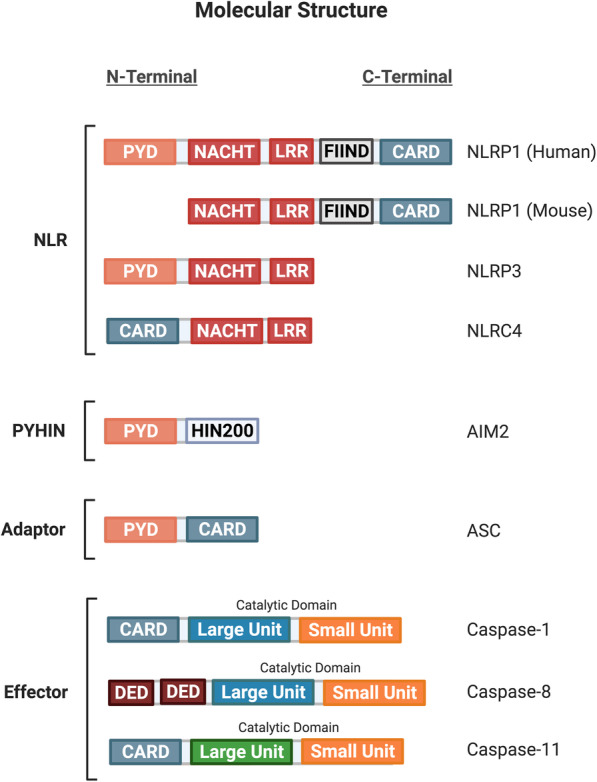
A schematic diagram illustrating the molecular structure of inflammasome receptor, adaptor and effector components. Members of the NLR family share similar structures: the NACHT domain is responsible for NLR oligomerization, while LRR is the inhibitory unit of the NACHT domain, keeping the receptor in its inactive state. The two other critical domains are the PYD domain and the caspase activation and recruitment domain (CARD) to facilitate interactions with other inflammasome components with similar domains to form the NLRP1, NLRP3 and NLRC4 inflammasome complex. Under the PYHIN family, the AIM2 inflammasome receptor has a PYD domain for adaptor binding and a HIN200 domain for dsDNA ligand binding. The adaptor protein ASC has both the PYD and CARD domain, serving as a linker protein between the inflammasome receptor and effector protein components. The three effector protein components share similar catalytic units (i.e. large and small units) and an N-terminal domain (i.e. CARD or DED) for inflammasome complex binding. Abbreviations: NLR, nucleotide-binding oligomerization domain-like receptor; NACHT, NAIP (neuronal apoptosis inhibitor protein) C2TA (class 2 transcription activator, of the MHC) HET-E (heterokaryon incompatibility) and TP1 (telomerase-associated protein 1); LRR, leucine-rich repeat; PYD, pyrin domain; CARD, caspase recruitment domain; NLRP1, NLR family pyrin domain containing 1; NLRP3, NLR family pyrin domain containing 3; NLRC4, NLR family CARD domain-containing protein 4; AIM2, absent in melanoma 2; HIN200, hematopoietic interferon-inducible nuclear proteins with a 200-amino-acid repeat; dsDNA, double-stranded DNA; ASC, apoptosis-associated speck-like protein containing a CARD; DED, death effector domain
