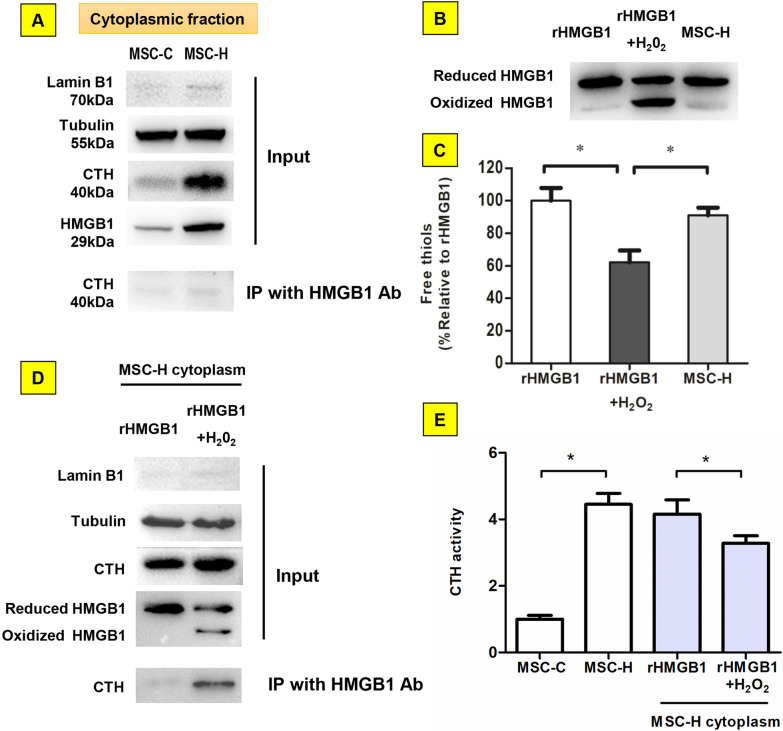
Fig. 4.
Regulation of γ-cystathionase (CTH) activity by HMGB1 upon oxidation. a Almost no CTH was co-immunoprecipitated by the bait protein HMGB1 from the cytoplasm of MSC-C and MSC-H cells. b, c The redox state of HMGB1 from the cytoplasm of MSC-H cells was analyzed by Western blot and protein free thiol assay. The partially oxidized HMGB1 sample with approximately 50% of HMGB1 oxidized (rHMGB1 + H2O2) was obtained by incubation the reduced HMGB1 standard (rHMGB1) with 50 μmol/l hydrogen peroxide for 1 h on ice. The immunoblotting by HMGB1 antibody yielded two bands of equal chemiluminescent intensity from the sample of rHMGB1 + H2O2 in comparison to a single band from rHMGB1. Intriguingly, the cytoplasmic extract from MSC-H cells was assayed to show a single band of HMGB1 corresponding to rHMGB1, suggesting cytoplasmic HMGB1 of MSC-H cells remained non-oxidized (B). HMGB1 was isolated from the cytoplasm of MSC-H cells by immunoprecipitation with anti-flag antibody. The purified HMGB1 contained high amount of free thiol, resembling rHMGB1 and significantly higher than the free thiol content of rHMGB1 + H2O2 (c). The MSC-H cytoplasm was mixed with rHMGB1 + H2O2 and rHMGB1, respectively, at 20:1 ratio of total protein. The HMGB1-associated protein was co-immunoprecipitated with HMGB1 antibody and analyzed by immunoblotting with anti-CTH antibody. A significant amount of CTH was co-immunoprecipitated from the mixture of MSC-H and rHMGB1 + H2O2. In comparison, co-immunoprecipitation yielded little CTH from the mixture of MSC-H and rHMGB1 (d). The CTH activity was determined by the efficiency of catalyzing α,γ-elimination reaction of L-cystathionine to produce α-ketobutyrate. The cytoplasm of MSC-H cells had a higher level of CTH activity than MSC-C cells. Adding rHMGB1 + H2O2 to MSC-H cytoplasm reduced the CTH activity, but rHMGB1 had no such effect (e). The asterisk indicated a P value < 0.05