Figure 5.
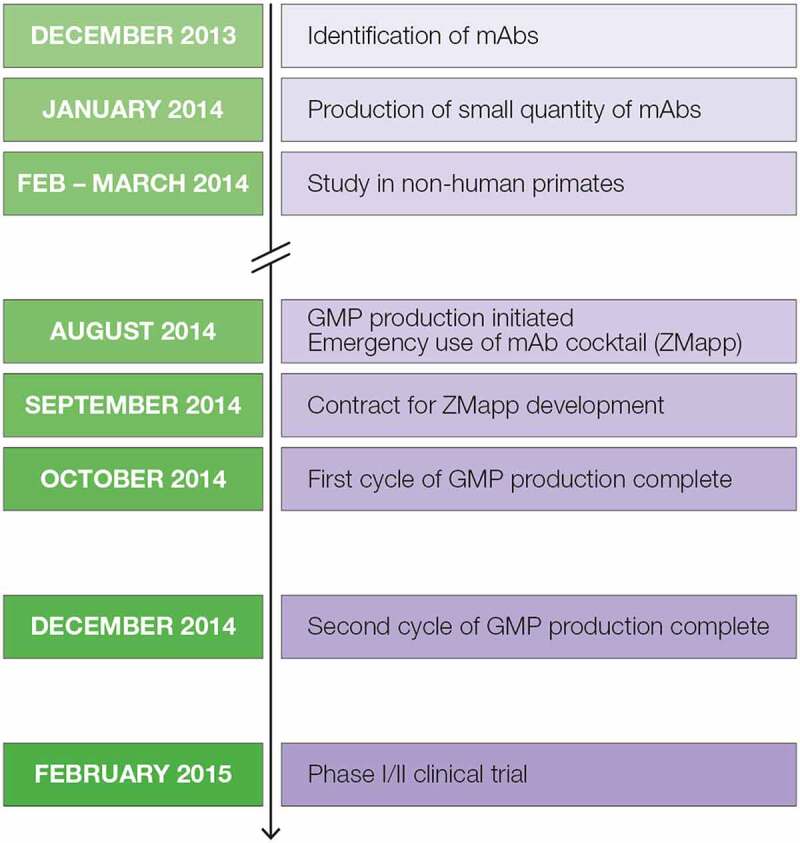
Timeline of development and production of the ZMapp antibody cocktail for emergency use and clinical trials for treating Ebola virus disease during the outbreak of 2014.7 Timeline representing development of the ZMapp antibody therapeutic. In December 2013 mAbs were identified, January 2014 small scale production initiated, February through March 2014 non-human primate studies performed, August 2014 GMP production initiated for emergency use of ZMapp, September 2014 a contract for ZMapp development initiated, October through December 2014 the first and second GMP production cycles completed, and February 2015 Phase I/II clinical trials begin.
