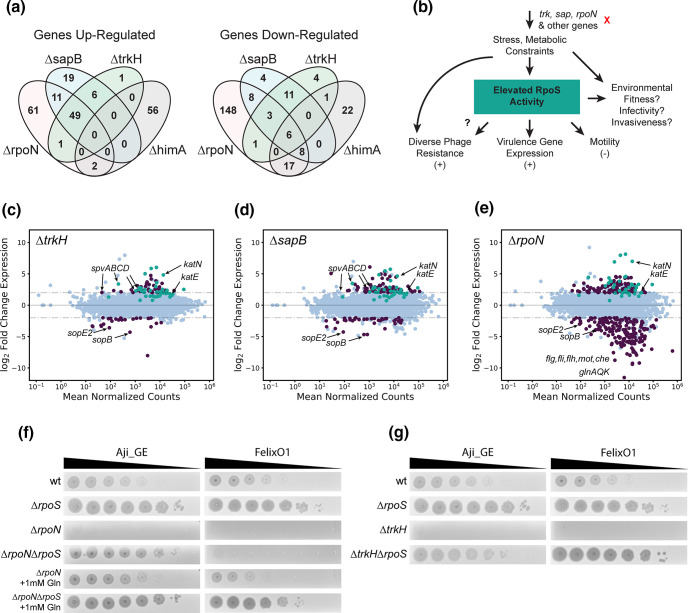
Fig. 4.
Cross-resistance mechanisms are mediated by RpoS. (a) Summary of genes with significant up- and down-regulation relative to wild-type for sapB, trkH, rpoN, and himA mutants. Reported values are genes with log2-fold changes over two and Bonferroni-corrected p values below 0.001. (b) Proposed model for phage cross-resistance observed in this study. Loss of function of genes such as trkH, sapB, or rpoN impose stress and metabolic constraints on S. Typhimurium. In some cases, this elevates RpoS activity and leads to multi-phage resistance. However, the environmental fitness, virulence, and invasiveness implications of these mutants are not known. (CDE) MA-plots for differential expression data for (c) MS1868∆trkH, (d) MS1868∆sapB, and (e) MS1868∆rpoN mutants over wild-type MS1868. Differentially expressed genes (abs(log2FC≥2), Bonferroni-corrected p values below 0.001) are shown in purple. RpoS-regulated genes are shown in teal based on a curated list from [120] . Specific genes are highlighted for emphasis including RpoS-activity indicators katE and katN. (f) Aji_GE and FelixO1 phage susceptibility assays focused on ∆rpoN-mediated phage resistance. For both phages supplementing with glutamine (Gln) restores phage infectivity in ∆rpoN context. A secondary deletion in rpoS is sufficient to restore Aji_GE infectivity in a ∆rpoN strain. However, FelixO1 is only restored with additional supplementation of glutamine. (g) Aji_GE and FelixO1 phage susceptibility assays focused on ∆trkH-mediated phage resistance. A secondary deletion in rpoS is sufficient to restore both Aji_GE and FelixO1 infectivity in a ∆trkH strain. Fig. 4a–e are created from Dataset S6 using Supplementary Code.