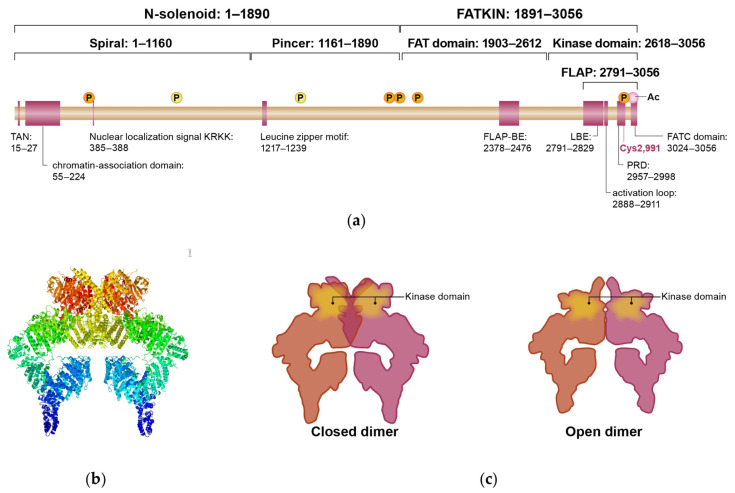
Figure 1.
(a) Map of the ATM protein, consisting of 3056 amino acids. ATM domains and motifs are listed with their amino acid numbers. Chromatin-association domain serves is important in interacting with chromatin or partner proteins. Nuclear Localization Signal enables nuclear translocation of ATM. Lys3016 is acetylated by TIP60. ATM has multiple phosphorylation sites that can substantially affect its kinase function. Ser367, Ser1893, Thr1885, Ser1981, and Ser2996 are auto-phosphorylated sites. Among them, auto-phosphorylation on Ser367, Ser1893, Ser1981, and acetylation on Lys 3016 are important for ATM activation. FATC domain on C-terminus of ATM is essential for its full activation. Cys2991 is essential to form disulfide bond between two ATM monomers. (b) The structure of an ATM closed dimer (PDB ID: 6K9L [23]), created with Jmol, an open-source Java viewer for chemical structures in 3D. (c) A schematic representation of the ATM protein. Interface of ATM homodimer consists of upper (FLAP−FLAP-BE) and lower (M-FAT−M-FAT) layers. In the closed dimer, active site of kinase domain is blocked, leaving ATM in an inactive state. In the open dimer, the upper interface is lost, resulting in more compact dimer and allowing partial access to the active site of the kinase domain. FAT-KIN: FAT-phosphatidylinositol 3-kinase-like kinase domain; FLAP-BE: FLAP-binding element; FLAP: Lst8 binding element (LBE), activation loop, and PIKK-regulatory domain (PRD) region.