Table 1.
Inhibitory activity of the synthesized compounds against class I HDACs.
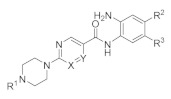
| Cpd. No. | X | Y | R 1 | R 2 | R 3 | HDAC1 (IC50 µM) |
HDAC2 (IC50 µM) |
HDAC3 (IC50 µM) |
HDAC8 (% Inhib.) |
|---|---|---|---|---|---|---|---|---|---|
| 19a | CH | N |
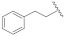
|
H | H | 0.51 ± 0.05 | 0.80 ± 0.07 | 1.12 ± 0.07 | 0% @ 1 µM |
| 19b | CH | N |
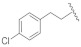
|
H | H | 25.8% @ 2 µM | 30.3% @ 2 µM | 65.2% @2 µM | 3.4% @ 1 µM |
| 19c | N | CH |
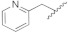
|
H | H | 33.9% @ 2 µM | 20.1% @ 2 µM | 26.8% @2 µM | 0% @ 1 µM |
| 19d | CH | N |
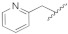
|
H | H | 0.52 ± 0.07 | 1.43 ± 0.08 | 1.06 ± 0.04 | 0% @ 1 µM |
| 19e | CH | N |
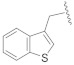
|
H | H | 0.21 ± 0.07 | 0.71 ± 0.04 | 0.84 ± 0.03 | 0% @ 1 µM |
| 19f | CH | N |
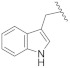
|
H | H | 0.13 ± 0.01 | 0.28 ± 0.01 | 0.31 ± 0.01 | 0% @ 1 µM |
| 19g | CH | N |
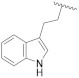
|
H | H | 0.31 ± 0.03 | 0.96 ± 0.05 | 0.49 ± 0.06 | 0% @ 1 µM |
| 19h | CH | N |
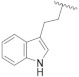
|
F | F | 0.81 ± 0.07 | 0.74 ± 0.03 | 0.57 ± 0.02 | n.d. |
| 19i | CH | N |
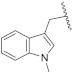
|
Cl | H | 3.0 ± 0.2 | 2.7 ± 0.2 | 1.9 ± 0.1 | n.d. |
| 19j | N | CH |
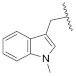
|
H | H | 0.45 ± 0.06 | 0.93 ± 0.04 | 1.75 ± 0.06 | 0% @ 1 µM |
| 19k | CH | N |
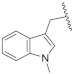
|
H | H | 0.14 ± 0.02 | 0.56 ± 0.04 | 0.59 ± 0.03 | 0% @ 1 µM |
| 19l | CH | N |
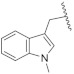
|
F | F | 0.29 ± 0.03 | 0.56 ± 0.02 | 0.81 ± 0.05 | n.d. |
| 19m | CH | N |
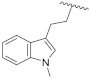
|
F | H | 0.40 ± 0.06 | 1.48 ± 0.19 | 0.40 ± 0.02 | n.d. |
| 19n | N | CH | CH3 | H | H | 5% @ 1 µM | 7% @ 1 µM | 13% @ 1 µM | n.d. |
| 19o | CH | N | CH3 | H | H | 27% @ 1 µM | 15% @ 1 µM | 30% @ 1 µM | n.d. |
| 21a | CH | N |
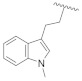
|
H | 2-Thienyl | 0.26 ± 0.01 | 2.47 ± 0.22 | 0% @ 1 µM | n.d. |
| 21b | CH | N |
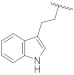
|
H | 4-F-C6H4 | 0.70 ± 0.08 | 0.77 ± 0.06 | 0% @ 1 µM | 0% @ 1 µM |
| 21c | CH | N |
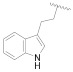
|
H | 2-F-C6H4 | 0.76 ± 0.07 | 0.76 ± 0.04 | 15 ± 1 | 0% @ 1 µM |
| 23a | CH | N | H | F | H | 3.30 ± 0.18 | 2.20 ± 0.20 | 0.40 ± 0.01 | 0% @ 1 µM |
| 23b | CH | N |
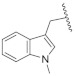
|
H | F | 0.27 ± 0.03 | 0.50 ± 0.03 | 0.50 ± 0.02 | 38% @ 1 µM |
| 23c | CH | N |
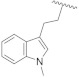
|
H | F | 0.33 ± 0.02 | 1.37 ± 0.08 | 0.59 ± 0.04 | n.d. |
| 25a | CH | N | H | Cl | H | 0% @ 1 µM | 0% @ 1 µM | 8.7 ± 0.4 | n.d. |
| 25b | CH | N | H | F | F | 4.3 ± 0.3 | 4.2 ± 0.10 | 1.6 ± 0.1 | n.d. |
| 27a | CH | N | H | H | CF3 | 0% @ 1 µM | 0% @ 1 µM | 0% @ 1 µM | n.d. |
| 27b | CH | N | H | CH3 | H | 0% @ 1 µM | 0% @ 1 µM | 0% @ 1 µM | n.d. |
| 27c | CH | N | H | OCH3 | H | 20.0 ± 1.0 | 14.0 ± 2.0 | 14.0 ± 1.0 | n.d. |
| 29a | CH | N | CH3 | H | 3-Thienyl | 0.11 ± 0.01 | 0.18 ± 0.06 | 4.4 ± 0.1 | n.d. |
| 29b | CH | N | H | H | 2-Thienyl | 0.07 ± 0.01 | 0.26 ± 0.01 | 6.1 ± 0.7 | n.d. |
| 29c | CH | N | H | H | 4-F-C6H4 | 0.16 ± 0.03 | 0.34 ± 0.01 | 6.7 ± 0.5 | n.d. |
| 29d | CH | N | CH3 | H | 2-F-C6H4 | 0.18 ± 0.01 | 0.26 ± 0.07 | 12.0 ± 1.0 | n.d. |
| CI994 | -- | -- | -- | -- | -- | 37% @ 1 µM | 36% @ 1 µM | 32% @ 1 µM | n.d. |
| RGFP-966 | -- | -- | -- | -- | -- | 16 ± 2 | 11 ± 1 | 1.3 ± 0.1 | n.d. |
| MS-275 | -- | -- | -- | -- | -- | 0.93 ± 0.1 | 0.95 ± 0.03 | 1.8 ± 0.1 | n.d. |
| Mocetinostat | -- | -- | -- | -- | -- | 0.33 ± 0.04 | 0.34 ± 0.01 | 0.93 ± 0.05 | n.d. |
n.d. not determined, -- no substituents.
