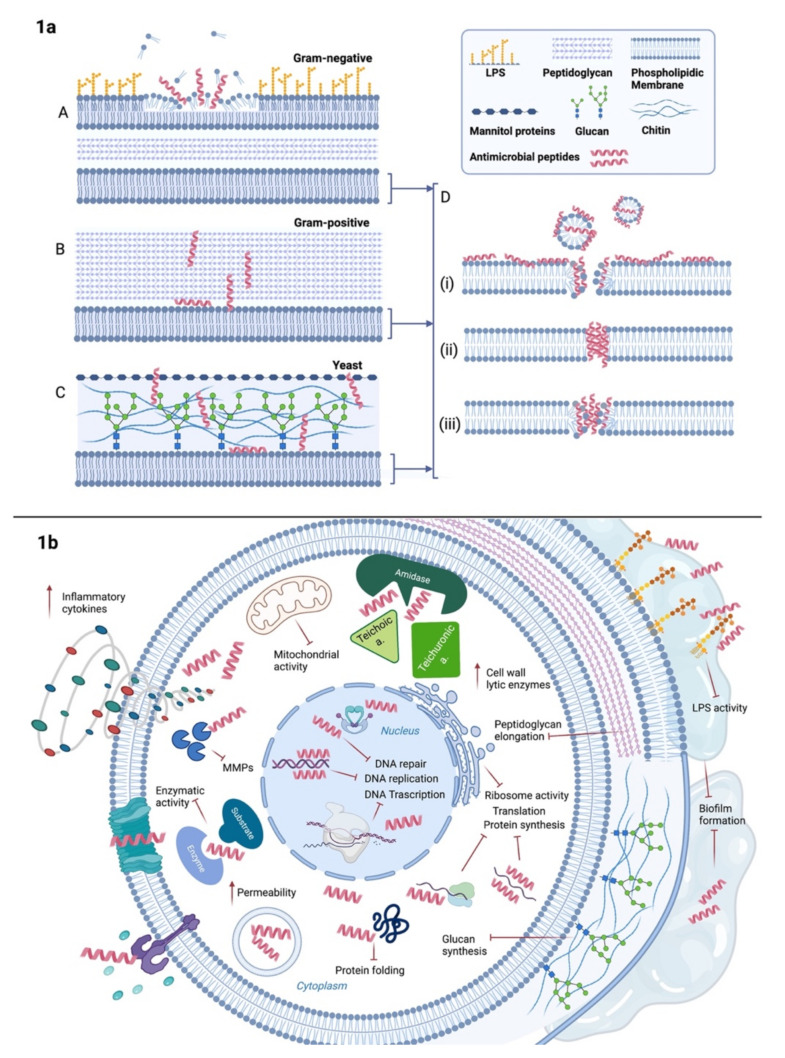
Figure 1.
AMPs broad-spectrum antimicrobial activity. (a) Primarily, AMPs’action is based on their action on cytoplasmic membranes, i.e., perturbation or disruption. However, in presence of Gram-negative bacteria (A) AMPs have to firstly cross the outer phospholipidic membrane and secondly traverse the peptidoglycan layer before reaching the inner membrane. In Gram-positive bacteria (B) they navigate through the thick cell wall of peptidoglycan and in fungi (C), they encounter mannitol proteins, glucans and chitin prior to access to the cytoplasmic membrane. Once reached the phospholipidic bilayer, they induce perturbation via pore formation following either (D) (i) carpet-like, (ii) barrel-stave or (iii) or toroidal pore model depending on the peptide composition. (b) Besides pore formation, some AMPs bind some components and receptors on the extracellular side of the membrane, i.e., Toll-like receptors; others manage to enter the cytosol through direct penetration in vesicles or channels thus destabilizing the permeability and activating the inflammatory cytokines cascade. Intracellularly, they could also interfere with DNA or RNA leading to degradation and cell death. They may also affect mitochondrial activity or protein synthesis by targeting ribosome subunits or protein folding. In the case of bacterial cell wall, they can prevent elongation of peptidoglycan chains or hinder teichoic and teichuronic binding acids to amidases. Cell wall components inhibition will promote cell autolysis. In the extracellular space, AMPs can sequestrate LPS reducing the impact of endotoxins on the host’s immune response. In fungal cells, AMPs can intervene on glucan synthesis thus blocking the building pieces of their wall. Further inhibitory action on biofilm matrix impairs the quorum sensing and improves the susceptibility of the single pathogens in both bacterial and fungal communities.