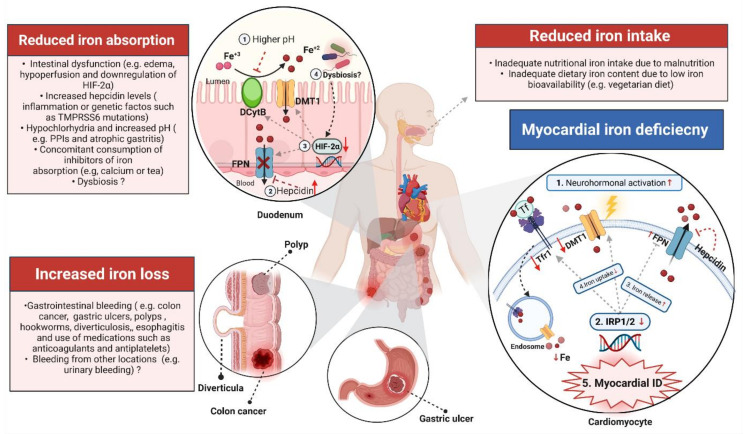
Figure 2.
Summary of the current understanding of the mechanisms underlying iron deficiency in heart failure. This step is essential to enable iron uptake by the divalent metal transporter (DMT1). This reduction process is influenced by the pH of the luminal contents, and thus, any factors that influence the pH, such as proton pump inhibitors, can impair non-haeme iron absorption. Within the enterocyte, iron can be stored in ferritin or exported into the bloodstream by the iron exporter ferroportin (FPN), which is controlled by hepcidin. Increased hepcidin levels internalise FPN, leading to sequestration of iron within the enterocytes and thus impairing absorption of iron to the blood. HIF-2 regulates transcription of DCytB, DMT1 and FPN iron transport machinery. Downregulation of HIF-2 can lead to a dysfunctional iron regulating system in HF. Gut microbial metabolites can decrease HIF-2 expression and thus may influence systemic iron homeostasis. The third mechanism that could also result in systemic ID is increased iron loss due to gastrointestinal pathology such as colon cancer. In the heart, neurohormonal activation leads to myocardial ID by downregulating iron-regulatory proteins (IRP1/2). Iron circulation in the heart is controlled by IRP1/2 and hepcidin. In turn, this defective downregulation of IRP1/2 as well as hepcidin leads to increased iron release (as a result of decreased hepcidin levels and thus higher FPN) and decreased iron uptake due to downregulation of transferrin receptor 1 and DMT1. (Created with BioRender.com, accessed on 24 November 2021).