Table 1.
Optimization of SOMOphilic alkynylation of alkenes a.
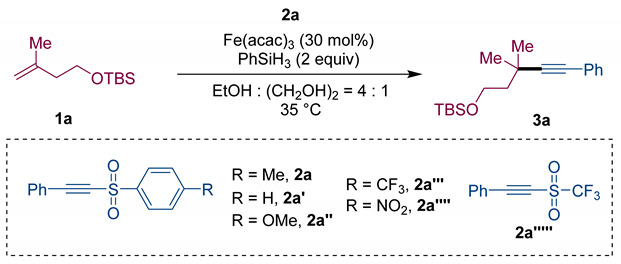
| Entry | Variation from the “Standard Conditions” | Yield (%) b |
|---|---|---|
| Entry 1 | none | 85 (81) c |
| Entry 2 | 2a′ instead of 2a | 80 (72) c |
| Entry 3 | 2a″ instead of 2a | 71 |
| Entry 4 | 2a‴ instead of 2a | 49 |
| Entry 5 | 2a⁗ instead of 2a | ND |
| Entry 6 | 2a′′′′′ instead of 2a | ND |
| Entry 7 | Only EtOH instead of EtOH and (CH2OH)2 | 62 |
| Entry 8 | Fe(acac)3 (20 mol%) instead of Fe(acac)3 (30 mol%) | 60 |
| Entry 9 | In(acac)3 instead of Fe(acac)3 | ND |
| Entry 10 | Co(acac)3 instead of Fe(acac)3 | ND |
| Entry 11 | FeCl3 instead of Fe(acac)3 | 45 |
| Entry 12 | 1a (0.2 mmol), 2a (0.3 mmol) | 65 |
a Standard conditions: 1a (0.3 mmol, 1.5 equiv), 2a (0.2 mmol, 1.0 equiv), Fe(acac)3 (30 mol%), PhSiH3 (0.4 mmol, 2.0 equiv), in EtOH (0.8 mL) and (CH2OH)2 (0.2 mL) at 35 °C for 12 h. b Determined by GC-MS using dodecane as the internal standard. c Isolated yield in parentheses.
