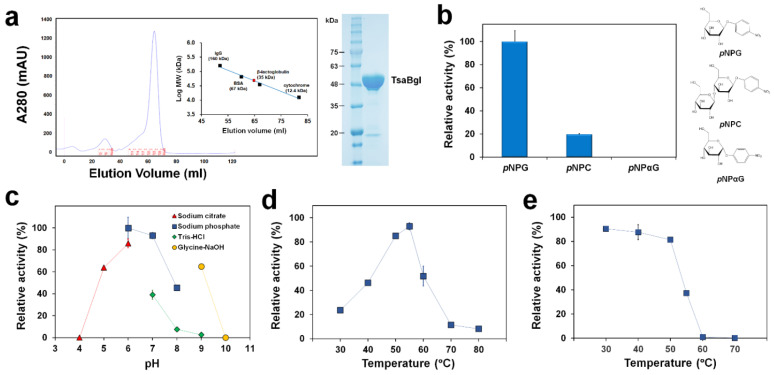
Figure 1.
Characterization of the β-glucosidase activity of TsaBgl. (a) Elution profile of TsaBgl on gel filtration chromato-graphy and purified protein. (b) Enzyme activity of TsaBgl towards various p-nitrophenyl substrates at 55 °C in 100 mM sodium phosphate (pH 6.0). (c) Enzyme activity of TsaBgl at various pH, ranging from pH 4 to 10, at 55 °C. (d) Enzyme activity of TsaBgl at various temperatures ranging from 30 to 80 °C in 100 mM sodium phosphate buffer (pH 7.0). Relative activity in panels (b–d) is defined as the enzyme activity relative to that obtained at pH 6.0 (100 mM sodium phosphate) and 55 °C with pNPG, set as 100%, with a specific activity of 23.6 U/mg. (e) Thermal stability of TsaBgl. The enzyme was pre-incubated at 30, 40, 50, 60, and 70 °C for 10 min without substrate, after which residual activities were measured under the standard conditions as detailed in the Materials and Methods section. The activity without pre-incubation was set as 100%, where the specific activity was 23.6 U/mg, and residual activities were calculated as its percentage. Error bars represent the standard deviations of three independent experiments.