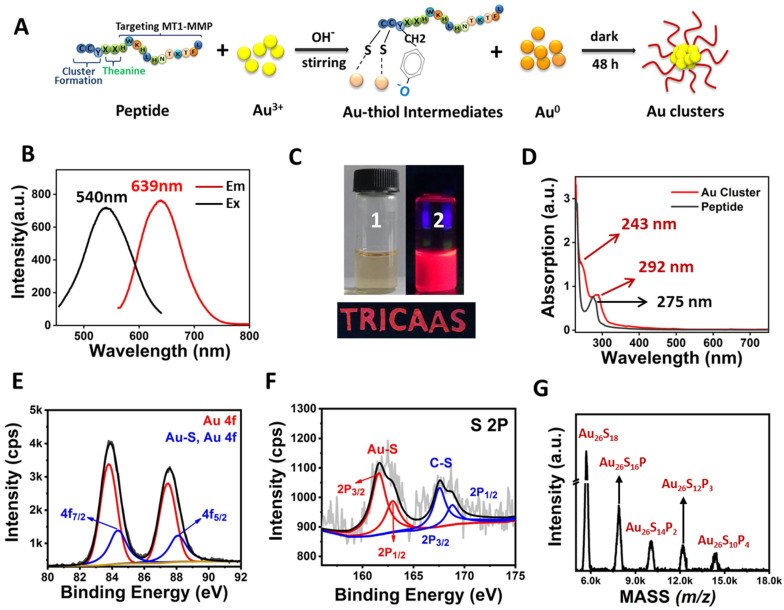
Figure 1.
Characterization of Au26 cluster. (A) The formation of Au clusters. (B) Fluorescence excitation and emission spectra of Au clusters. (C) Photo images of Au clusters under visible (1) and a portable UV lamp (2). The excellent fluorescence properties of Au clusters can make them be used as fluorescent inks. e.g., “TRICAAS” was written using Au clusters emitting red fluorescence under a portable UV lamp. “TRICAAS” is the abbreviation of our affiliation institution. (D) Absorption spectra of peptide and Au clusters. (E,F) XPS spectrum of Au 4f and S 2P of Au clusters. (G) MALDI-TOF-MS spectrum of Au clusters. The mass of the Au26P9 molecule fragment could be written by Au26PxS18–2x, where x ranges from 0 to 9, P represents the peptide, and S represents sulfur.