Abstract
Plant secondary metabolites (PSMs) are vital for human health and constitute the skeletal framework of many pharmaceutical drugs. Indeed, more than 25% of the existing drugs belong to PSMs. One of the continuing challenges for drug discovery and pharmaceutical industries is gaining access to natural products, including medicinal plants. This bottleneck is heightened for endangered species prohibited for large sample collection, even if they show biological hits. While cultivating the pharmaceutically interesting plant species may be a solution, it is not always possible to grow the organism outside its natural habitat. Plants affected by abiotic stress present a potential alternative source for drug discovery. In order to overcome abiotic environmental stressors, plants may mount a defense response by producing a diversity of PSMs to avoid cells and tissue damage. Plants either synthesize new chemicals or increase the concentration (in most instances) of existing chemicals, including the prominent bioactive lead compounds morphine, camptothecin, catharanthine, epicatechin-3-gallate (EGCG), quercetin, resveratrol, and kaempferol. Most PSMs produced under various abiotic stress conditions are plant defense chemicals and are functionally anti-inflammatory and antioxidative. The major PSM groups are terpenoids, followed by alkaloids and phenolic compounds. We have searched the literature on plants affected by abiotic stress (primarily studied in the simulated growth conditions) and their PSMs (including pharmacological activities) from PubMed, Scopus, MEDLINE Ovid, Google Scholar, Databases, and journal websites. We used search keywords: “stress-affected plants,” “plant secondary metabolites, “abiotic stress,” “climatic influence,” “pharmacological activities,” “bioactive compounds,” “drug discovery,” and “medicinal plants” and retrieved published literature between 1973 to 2021. This review provides an overview of variation in bioactive phytochemical production in plants under various abiotic stress and their potential in the biodiscovery of therapeutic drugs. We excluded studies on the effects of biotic stress on PSMs.
Keywords: secondary metabolites, climate change, drug discovery, abiotic stress
1. Introduction
Plant secondary metabolites (PSMs) are small molecules with diverse chemical structures and biological activities. Unlike primary metabolites, which are the main drivers of essential life functions, including cell formation, PSMs are neither necessary for primary life functions nor possess high-energy bonds [1]. However, PSMs play essential secondary physiological and biochemical functions that ensure plant fitness and survival, particularly concerning their interactions with the environment and coping with biotic and abiotic stress [1]. These factors, especially abiotic stressors (nutrient deficiencies, seasons, salinity, wounding, drought, light, UV radiation, temperature, greenhouse gases, and climate changes), cause significant perturbations in chemotypes and levels of PSMs production. For example, plants produce more terpenoids when exposed to high temperatures [2], and UV-B (280–315 nm) radiation induces tree foliage to produce more phenolic acids and flavonoids as protective pigments [3,4]. Phenolics and flavonoids are well-known for their antioxidative and anti-inflammatory properties [5,6,7]. Similarly, the production of antioxidative compounds such as glutathione, g-aminobutyric acid (GABA), terpenoids, and volatile organic compounds (VOCs) increases under elevated O3 [8].
PSMs are vital for human health and form many pharmaceutical drugs’ backbone. Indeed, more than 25% of the existing drugs belong to PSMs [9]. The most popular PSMs-derived drugs are morphine (isolated from Papaver somniferum), digitoxin (isolated from Digitalis purpurea), taxol (isolated from Taxus baccata), artemisinin (isolated from Artemisia annua) and quinine (isolated from Cinchona officinalis), vinblastine and vincristine (isolated from Catharanthus roseus); and aspirin (first isolated as salicylic acid from Filipendula ulmaria). Since plants exposed to various abiotic stress conditions produce many PSMs in higher concentrations as their coping mechanism [10,11,12], it presents opportunities for natural product researchers and pharmaceutical companies to explore the biochemical responses of plants to climatic stress for developing many novel therapeutics. However, there is no comprehensive literature review examining the scope of plants affected by abiotic stresses for drug discovery.
Therefore, this scoping review examines recent advances related to PSMs in plants affected by abiotic stress/or abiotic growth factors, their roles as protective phytochemicals, and their potential for novel drug lead compounds. Although primary metabolites such as carbohydrates [13,14] and peptides [15,16] are also known to play roles in the plant’s defense response, our review focuses on selected classes of PSMs, including flavonoids, terpenoids, alkaloids, saponins, tannins, and cyanogenic glycosides. We have collected published information on plants affected by abiotic stresses (primarily studied in the simulated growth conditions) and their PSMs (including pharmacological activities) from PubMed, Scopus, MEDLINE Ovid, Google Scholar, Databases, and journal websites using the following keywords: “stress-affected plants,” “plant secondary metabolites,” “bioactive compounds,” “abiotic stress,” “climatic influence,” “pharmacological activities,” “drug discovery,” and “medicinal plants.” We have retrieved published literature between 1973 to 2021 (only related to PSMs produced under ex situ growth conditions), analysed the content, and presented the information in the form of figures and tables. The chemical structures were drawn by using ChewDraw Professional software, and each structure was cross-checked for their correctness using ChemSpider and HMDB databases. We excluded studies on the effects of biotic stress on PSMs.
2. Plant Secondary Metabolites and Their Biological Roles
Generally, all plants produce secondary metabolites for defense, attraction, communication, and mediating stress [17]. For example, plants produce VOCs as defense molecules, and they are known to function as antimicrobial and insect repellent agents [18]. More than 200,000 PSMs have been identified [19], and with more than 391,000 plant species known worldwide [20], there is space for more discoveries. Some PSMs are specific to certain related plant taxa [21], and their concentrations can vary between populations and individual plants with plant ontogeny and tissue type [22,23]. These PSM variations can be due to genetic variability, but their concentrations are affected by environmental abiotic factors (growth conditions) such as those expected to intensify with climate change (e.g., heat stress, drought, UV radiation, and O3) [24], and herbivore and pathogen attacks [25,26]. Based on a biosynthetic pathway and chemical structure, PSMs have broadly been categorized into three major groups: (i) terpenoids (plant volatiles, sterols, carotenoids, saponins, and glycosides), (ii) phenolic compounds (flavonoids, phenolic acids, lignin, lignans, coumarins, stilbenes, and tannins), and (iii) nitrogen-containing compounds (alkaloids, glucosinolates, and cyanogenic glycosides) [27,28,29,30].
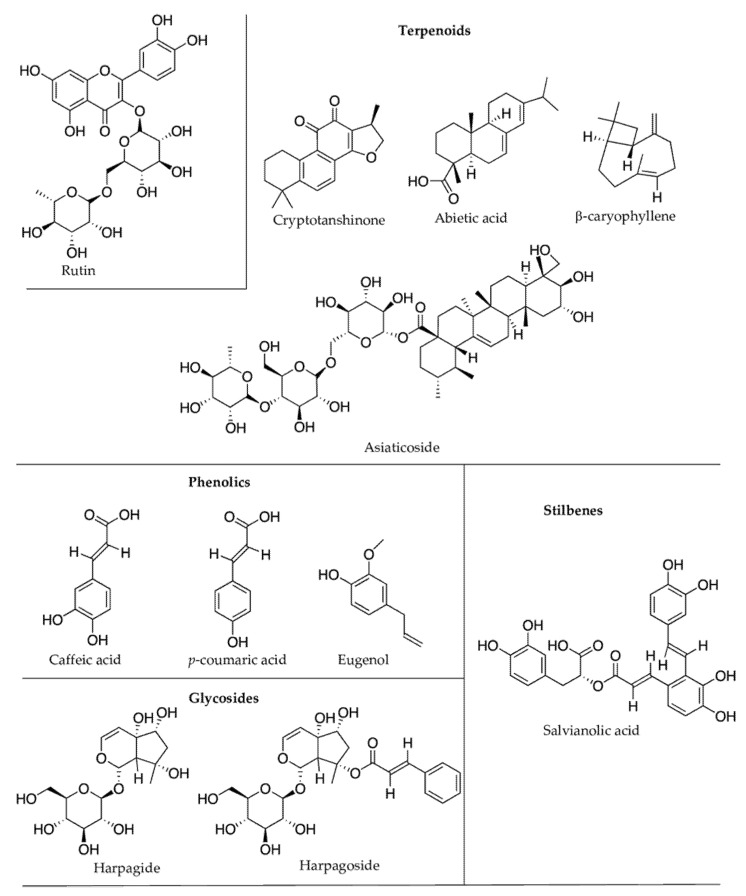
2.1. Terpenoids
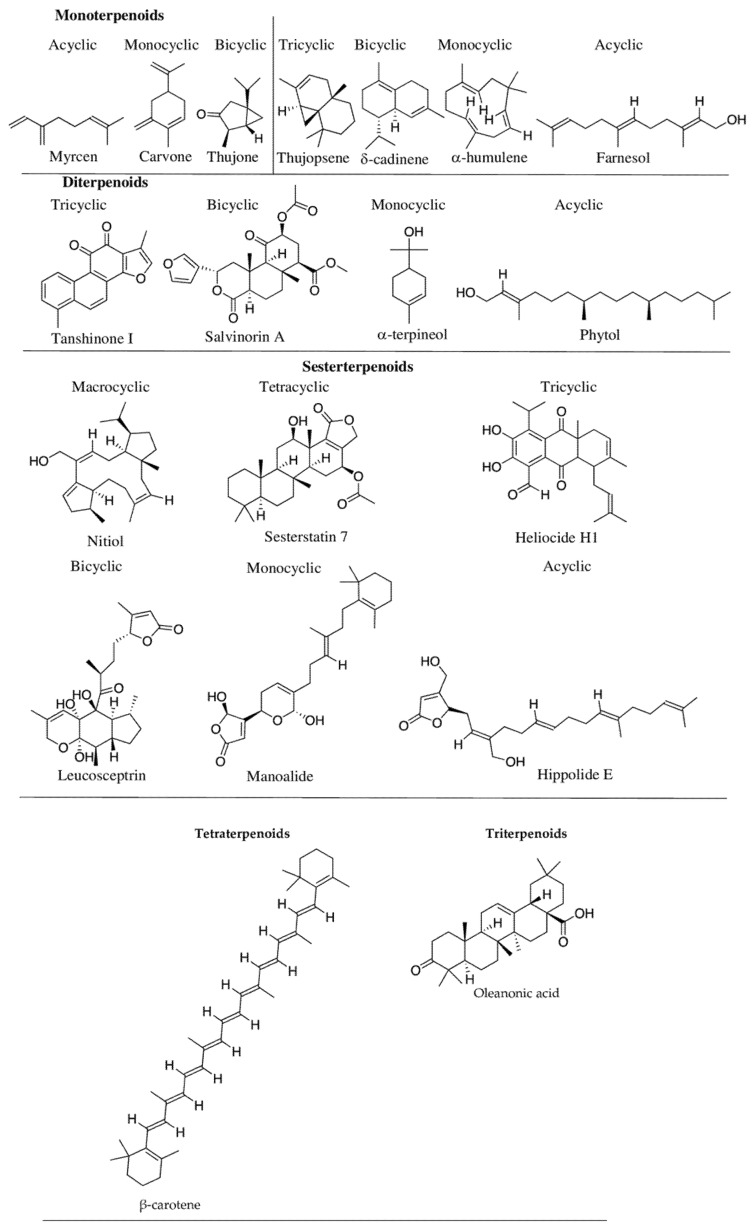
Terpenoids or isoprenoids are one of the most structurally diverse naturally occurring PSMs, with the main skeleton consisting of five-carbon isopentyl units, called 2-methyl-1,3-butadiene, or isoprene. Terpenes contain only isoprene units, while terpenoids have additional functional groups, such as ketone or heterocyclic and hydroxyl rings. Based on structural construction, terpenoids can be considered as two types, aliphatic (e.g., geraniol) and cyclic (e.g., limonene) terpenoids. Since terpenoids contain many isoprene units, they are divided into various groups, as described below (Figure 1):
Monoterpenoids—two isoprene units (C-10 carbon atoms,)—e.g., linalool;
Sesquiterpenoids—three isoprene units (C-15 carbon atoms)—e.g., β-caryophyllene;
Diterpenoids—four isoprene units (C-20 carbon atoms)—e.g., abietic acid;
Sesterterpenoids—five isoprene units (C-25 carbon atoms)—e.g., ophiobolin A;
Triterpenoids—six isoprene units (C-30 carbon atoms)—e.g., ganoderic acid;
Tetraterpenoids—eight isoprene units (C-40 carbon atoms)—e.g., α-carotene;
Polyterpenoids—more than eight isoprene units (>C-40 carbon atoms)—e.g., trans-1,4-polyisoprene.
Figure 1.
Representative examples of terpenoid plant secondary metabolites.
Terpenoids are formed from the mevalonate pathway inside cytosol or the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway inside the plastid [31]. The biosynthetic precursors of terpenoids include geranyl diphosphate (GPP) for monoterpenes; farnesyl diphosphate (FPP) for sesquiterpenes; and geranylgeranyl diphosphate (GGPP) for diterpenes [32]. An in-depth discussion on terpenoids’ biosynthetic pathway and structural diversity is covered by Aharoni et al. (2005) and Song et al. (2014) in their review [31,32]. More than 30,000 terpenes have been reported to date [33]. They are mostly phytohormones (e.g., gibberellins), photosynthetic pigments (e.g., phytol, carotenoids such as α-carotene and β-carotene), and carriers (e.g., ubiquinone, plastoquinone) in the electron chain transport systems [34,35]. The role of terpenoids is to protect plants directly (e.g., releasing phytoalexins after pathogen attacks) or indirectly by producing mixtures of volatile organic compounds (VOCs) to attract carnivores of their herbivores [36]. Phytoalexins are antimicrobial compounds produced after microbes’ challenge plants, and it is reviewed in-depth by González-Lamothe et al. [37]. VOCs include terpenoids (isoprene or hemiterpenoids and monoterpenoids), alkanes, alkenes, carbonyls, alcohols, esters, ethers, and acids [18]. VOCs are involved in plant-plant or plant-insect interactions, but some terpenoids act as lipid-soluble antioxidants inducing resistance to stress [38].
Several terpenoids have shown defensive roles against biotic and abiotic stresses in plants. Dahham et al. (2015) [39] and Porres-Martínez et al. (2016) [40] reported antioxidant activities of terpenes (sesquiterpene b-caryophyllene, and monoterpenes 1,8-cineole and α-pinene, respectively), suggesting their function in overcoming abiotic-induced oxidative stress. Terpenoids are also reported to protect plants from photodamage and oxidative stress by supporting photorespiration [41]. Carotenoids are the best-known terpenoids involved in photoprotection [42]. Other examples of defensive terpenes in plants are triterpene glycosides (or saponins) such as α-tomatine in the fruits and leaves of tomatoes [43] and avenacin in oat (Avena sativa) roots [44]. Avenacin and α-tomatine are important pre-formed antimicrobial compounds, commonly referred to as phytoanticipins, and they have defensive roles against microbial attacks [37]. Saponins are another group of compounds under terpenoids with triterpenes or steroidal aglycones linked to one or more sugar chains [45], but some saponins such as steroidal glycoalkaloids have a nitrogen atom in their aglycone chemical structure [45,46]. Similarl to many other PSMs, the amount and distribution of saponins in plants are influenced by season, biotic and abiotic stresses, and plant developmental stage. For example, maximum saponin production in Phytolacca dodecandra L’Hér. [47] and Dioscorea pseudojaponica Yamamoto [48] occurs during fruit and tuber development to prevent fruit loss and enable seed maturation. Under stress conditions, saponin levels in plants increase through jasmonate and salicylate signaling pathways [45].
2.2. Phenolic Compounds
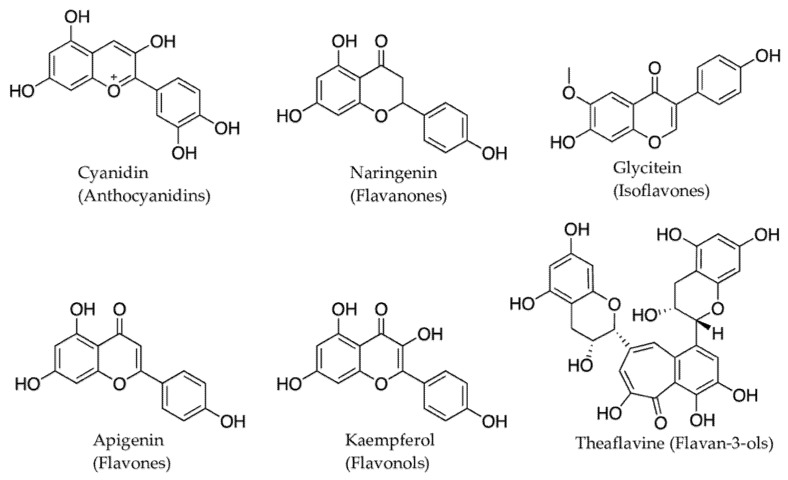
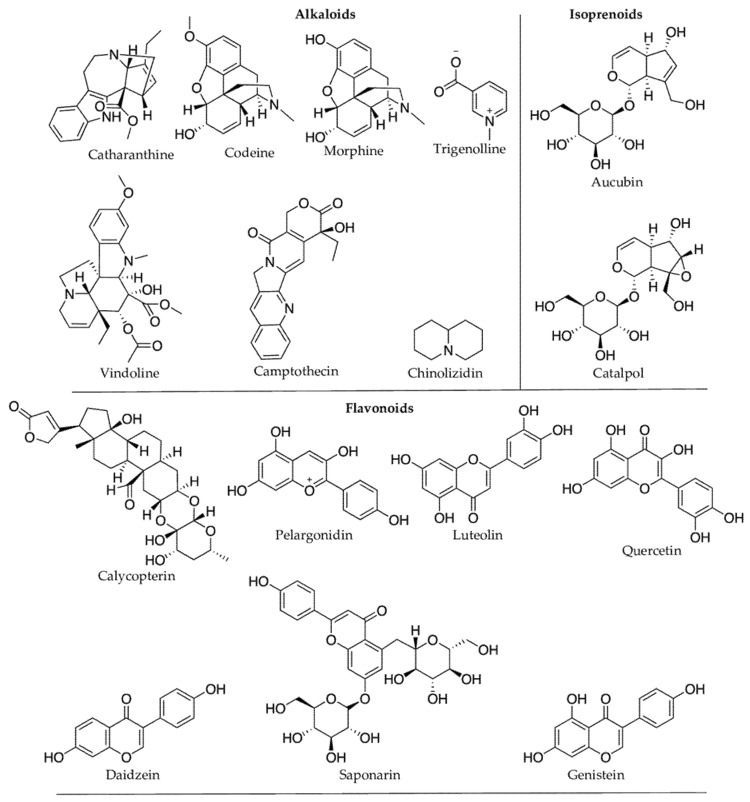
More than 8000 phenolic compounds are reported from plants, of which half of them are flavonoids (approximately 4000–4500 compounds) such as aglycone, glycosides, and methylated derivatives [6,49]. Phenolics exhibit diverse structures from single aromatic rings (e.g., in phloroglucinol, gentisic acid, ferulic acid, caffeic acid, and vanillin) to complex polymeric structures such as in lignins (e.g., coniferyl alcohol), coumarins (e.g., scopoletin), phenolic quinones (e.g., juglone), tannins (e.g., ellagic acid), and flavonoids [50,51]. Among phenolic group compounds, flavonoids are the most abundant, and stilbenes and lignans are less common.
Flavonoids are a diverse secondary metabolite group with a wide array of functions, including protection against stress. Flavonoids comprise seven sub-groups (Figure 2) (flavones, flavonols, flavanones, isoflavonoids, flavan-3-ols or catechins, and anthocyanins) [52,53] based on the C-ring carbon to which B-ring is attached, and also based on the degree of oxidation and unsaturation of their C-ring [53]. Flavones contain a double bond between positions 2 and 3 and a ketone functional group in position 4 of the C-ring. In comparison, flavonols have a hydroxy group at position 3 of the C-ring and are sometimes glycosylated. Unlike flavones, flavonones are saturated with a double bond between positions 2 and 3 of the C-ring. Flavan-3-ols have a hydroxyl group in position 3 of the C-ring, but there is no double bond between positions 2 and 3 [53]. Although the metabolic role of phenolics is not well-defined, their protective functions in plants are attributed to their ability to scavenge free radicals and filter harmful UV radiations [54,55]. Ferulic acid, caffeic acid, and p-coumaric acid (hydroxycinnamic acid derivatives) are some of the best-known UV-B attenuators in plants [56]. Flavonoids help plants adjust to extreme heat and cold [57] through increasing accumulation. When Schulz et al. (2016) [58] analyzed the expression of flavonoids in 20 mutants of two different Arabidopsis thaliana accessions (Col-0 and Ler) in response to freezing and cold acclimation (14 days at 4 °C), 19 mutants, which are gene-knock outs, did not exhibit flavonoid biosynthesis, with an exception to pap1-D mutant. A similar observation of increasing concentrations in flavonoids (anthocyanins and flavonols) was also reported by Pastore et al. (2017) [59] in grapevine berries, but tannins did not show any changes. The role of flavonoids in UV protection is also supported by Bieza and Lois’ work [60], in which they have isolated an Arabidopsis mutant tolerant to high levels of UV-B radiations. Such protective flavonoids are reported more in plants thriving in colder climates at higher elevations and semi-arid environments [61]. Flavonoid-based plant pigments, such as anthocyanins synthesized in the last step of the flavonoid biosynthesis pathway under UV stress upon acylation, can absorb UV radiation and scavenge ROS [62,63]. If not kept under control, ROS can cause direct damage to plants through the oxidation of essential biomolecules, leading to the accumulation of more ROS and ultimately programmed cell death [64].
Figure 2.
Representative examples of different subgroups of flavonoids: a major phenolic group of secondary metabolites.
Flavonoids such as quercetin can chelate transition metals (for example, Fe), consequently inhibiting Fenton reaction (conversion of H2O2 to toxic OH• radical), thereby creating a robust antioxidative environment in the plants [65]. Phenolics are also known to play a strategic role in reproduction as frugivore attractants that promote seed dispersal (e.g., anthocyanidins and anthocyanins such as cyanidin-3-glucoside) [66,67].
2.3. Nitrogen-Containing Compounds
2.3.1. Alkaloids
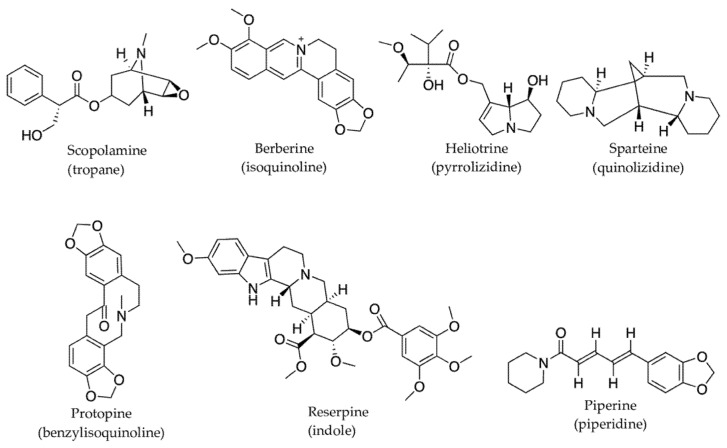
Alkaloids are the major group of plant defense molecules that contain a nitrogen atom(s) derived from the decarboxylation of amino acids and are known to occur in 20% of plant species [32]. There are seven types of alkaloids based on their amino acid precursors (Figure 3). Tropane, pyrrolidine, and pyrrolizidine alkaloids are derived from ornithine amino acid precursors; benzylisoquinoline from tyrosine amino acid precursors; indolequinoline from tryptophane amino acid precursors; and quinolizidine and piperidine alkaloids from lysine amino acid precursors [68]. Alkaloids are widely distributed among plant lineages and are particularly abundant in angiosperms. Individual plant species may contain fewer than five to more than 30 alkaloids (e.g., 74 alkaloids in Catharanthus roseus, 54 in Strychnos toxifera, and 39 in Rauwolfia serpentina) [68,69]. Generally, a plant family produces only one type of alkaloid, although a few families such as Solanaceae and Rutaceae accumulate a broad spectrum of alkaloids [70]. For example, Duboisia myoporoides R.Br. contains both a tropane alkaloid (hyoscine) and a pyridine alkaloid (nicotine) [71]. More than 20,000 alkaloids have been isolated, of which about 600 are known to be bioactive [72], but the exact physiological or metabolic role of alkaloids in plants remains poorly understood [68]. Alkaloids are best known for their defensive role as insect-herbivore deterrents owing to their characteristic bitter taste [73]. Thus, according to Levin [69], most alkaloid-bearing plants are found in the tropics, where intensive herbivore pressure is present. Defensive or toxic alkaloids in plants may be produced either by the plants themselves or by their symbiotic partners [74,75]. For example, the symbiotic endophyte Epichloe coenophiala in tall fescue grass [Lolium arundinaceum (Schreb.) Darbysh, syn. Festuca arundinacea (Schreb.), and Schedonorus arundinaceus (Schreb.) Dumort.) produces insecticidal alkaloids, lolines, and ergot, which cause ‘fescue toxicosis’ in grazing animals [76]. Alkaloid biosynthesis in plants is genetically controlled, but environmental factors such as light (UV), temperature, moisture, and soil nutrients also influence the type and rate of alkaloid production [76,77].
Figure 3.
Representative examples of seven different types of alkaloids produced in plants and their chemical structure.
2.3.2. Cyanogenic Glycosides and Glucosinolates
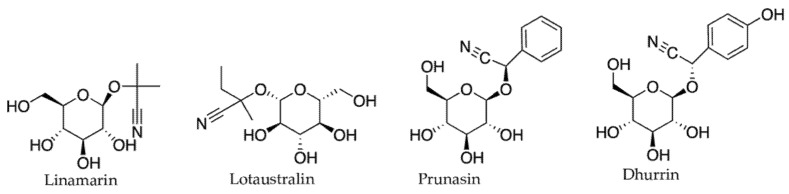
Other N-containing defense compound groups include cyanogenic glycosides and glucosinolates. These two groups are also derived from amino acid precursors and are significantly less diverse in their structure, with over a hundred compounds known from each group. Cyanogenic glycosides are reported in more than 2500 plant species [78], including ferns, gymnosperms, and angiosperms, while glucosinolates have been reported only in the order Capparales and in the genus Drypetes of the Euphorbiaceae [79]. According to Vetter [78] and Gleadow and Moller [80], some of the widely distributed cyanogenic glycosides in the plant kingdom are linamarin and lotaustralin (in Compositae, Linaceae, Fabaceae, Papaveraceae, and Euphorbiaceae); prunasin (in Myrtaceae, Polypodiaceae, Rosaceae, Saxifragaceae, Scrophulariaceae, and Myoporaceae); and dhurrin (in Poaceae and Euphorbiaceae) (Figure 4). Important food crops such as apple (Malus domestica), apricot (Prunus armeniaca), bamboo (Bambusa vulgaris), cassava (Manihot esculenta), cocoyam (Colocasia esculenta and Xanthosoma sagittifolium), and sorghum (Sorghum bicolor) are known to contain cyanogenic glycosides [81,82]. Cyanogenic glycosides and glucosinolates are generally higher in young leaves [83,84] and reproductive tissues [23,83,84,85]. They are toxic in higher concentrations [86], but in response to the low light, some plants such as tropical Prunus turneriana tend to accumulate more cyanogenic glycosides in older leaves. Although cyanogenic glycosides and glucosinolates in plants also respond to climatic stress such as drought and increased temperatures [80,86], they are not discussed in the following sections of this review.
Figure 4.
Examples of widely distributed cyanogenic glycosides in plant kingdom.
3. Factors Influencing PSMs Production in Plants
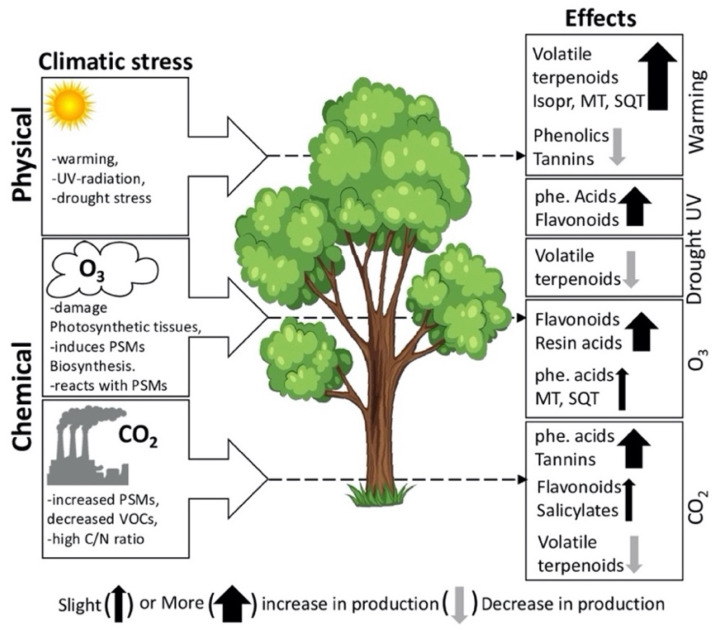
Vickers et al. [87] have proposed two hypothetical mechanisms by which plants may respond to multiple external stressors: membrane stabilization and direct antioxidative scavenging of reactive oxygen species (ROS) generated under stressful conditions and to attract pollinators [88]. Under oxidative stress, plants either directly catalyze ROS to less harmful compounds using enzymes such as superoxide dismutase, catalase, and peroxidase or mediate enzymatic regeneration of antioxidants (e.g., monodehydroascorbate reductase, dehydroascorbate reductase, and glutathione reductase) [64]. Non-volatile isoprenoids such as tocopherols, zeaxanthin, and carnosic acid can scavenge ROS directly by reactions through hydroxyl radicals [89,90]. Interestingly, rising global temperatures and other environmental variables such as atmospheric O3 concentration and UV-B radiation are known to increase plant stress and, therefore, enhance or limit PSMs production as means to cope with such stressors. VOC emissions from plants are triggered by wounding and tri-trophic interactions (plant-herbivorous-carnivorous arthropods) [91] and they are influenced by various environmental factors, including temperature, light, moisture, and pollutants [92]. Individual stress has a selective influence on PSMs production, either by inducing or inhibiting the compound biosynthesis or emission based on stress conditions in plants (Figure 5). While PSMs have diverse functions in plants, their production also depends on multiple factors [34,93]. The effects of abiotic stress on PSMs production are given in Table 1.
Figure 5.
Abiotic stresses and their influence on the types of secondary metabolites in plants (adapted from [94,95,96,97]). Abbreviations: UV radiation = ultraviolet radiation; PSMs = plant secondary metabolites; O3 = ozone; CO2 = carbon dioxide; Isopr = isoprenoids; MT = monoterpenes; SQT = sesquiterpenes; phe. acids = phenolic acids.
Table 1.
Plant secondary metabolites produced in response to abiotic stresses and their reported pharmacological properties.
| Stress Condition(s) | Plant Species (Family) | PSMs Produced | Effects on PSMs Concentration | Compound Class | Bioactive Compounds | Reported Pharmacological Properties |
|---|---|---|---|---|---|---|
| Cold stress | Catharanthus roseus (Apocynaceae) [98] | vindoline | Decrease | Alkaloids | vindoline | Antidiabetic [99] |
| Cold stress | Glycine max (Fabaceae) [94] | genistein, daidzein | Increase | Phenolics | genistein, daidzein | Antiproliferative [95,96] |
| Cold stress | Solanum lycopersicon (Solanaceae) [87,97] | (Z)-3-hexenol and (E)-2-hexenal (dominant); 1-hexanol and 1,4-hexadienal (smaller quantities) | Increase | Fatty Acyls | (E)-2-hexenal | Antibacterial [100] |
| Cold stress | β-phellandrene, (E)-β-ocimene | Increase | Terpenoids | NA | NA | |
| Cold stress | δ-elemene, α-humulene and β-caryophyllene (dominant); in severe cold: β-elemene is produced. | Increase | Terpenoids | δ-elemene, α-humulene and β-caryophyllene | Antiproliferative [101]; anticancer [102]; anti-inflammatory [103] | |
| Cold stress | Zea mays (Poaceae) [104] | pelargonidin | Increase | Phenolics | pelargonidin | Antithrombotic [105] |
| Cold stress | Fagopyrum tartaricum (Polygonaceae) [106] | anthocyanins (e.g.,3-O-galactosides) and anthocyanidins (e.g., malvidin) | Increase | Phenolics | anthocyanins | Antioxidant [107] |
| Cold stress | Withania somnifera (Solanaceae) [108] | withanolide A, withaferin A | Increase | Terpenoids | withanolide A; withferin A | Neuroprotective [109]; anticancer [110] |
| Cold stress | Camellia sinensis (Theaceae) [111] | nerolidol glucoside | Increase | Terpenoids | NA | NA |
| Drought | Amaranthus tricolor (Amaranthaceae) [112] | hydroxybenzoic acids (gallic acid, vanillic acid, syringic acid, p-hydroxybenzoic acid, salicylic acid, ellagic acid), hydroxycinnamic acids (caffeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, m-coumaric acid, sinapic acid, trans-cinnamic acid), flavonoids (iso-quercetin, hyperoside, rutin). | Increase | Phenolics (Flavonoids) | p-hydroxybenzoic acid | Antisickling activity [113] |
| Drought | Camellia sinensis (Theaceae) [114] | Epicatechins | Increase | Phenolics (Flavonoids) | epicatechins | Antioxidant [115] |
| Drought | Camptotheca acuminata (Nyssaceae) [116] | camptothecin | Increase | Alkaloids | camptothecin | Antitumour [117] |
| Drought (PEG-induced) | Catharanthus roseus (Apocyanaceae) [118] | vinblastine | Increase | Alkaloids | vinblastine | Anticancer [119] |
| Drought | Cistus clusii (Cistaceae) [120] | epigallocatechin gallate, epicatechin, epicatechin gallate, and ascorbic acid. | Increase | Phenolics (Flavonols) | epigallocatechin gallate | Anticancer [121]; antibacterial [122] |
| Drought | Crataegus laevigata, C. monogyna (Rosaceae) [123] | chlorogenic acid, catechin, (−)-epicatechin | Increase | Phenolics | chlorogenic acid, (−)-epicatechin | Antioxidant [124,125] |
| Drought | Glycine max (Fabaceae) [126] | trigonelline | Increase | Alkaloids | trigonelline | Antidiabetic [127] |
| Drought | Hypericum brasiliense (Hypericaceae) [128] | isouliginosin B, rutin, 1,5-dihydroxyxanthone | Increase | Phenolics | isouliginosin B, rutin, | Antinociceptive [129]; Anticancer [130] |
| betulinic acid | Terpenoids | betulinic acid | Anticancer [131] | |||
| Drought | Lupinus angustifolius (Fabaceae) [132] | chinolizidin | Increase | Alkaloids | NA | NA |
| Drought | Papaver somniferum (Papaveraceae) [133] | morphine, codeine | Increase | Alkaloids | morphine, codeine | Analgesic [134,135] |
| Drought | Pinus sylvestris (Pinaceae) [136] | abietic acid | Increase | Terpenoids | abietic acid | Antiallergic [137]; anti-inflammatory [138] |
| Drought | Salvia miltiorrhiza (Lamiaceae) [139] | tanshinones, cryptotanshinone | Increase | Terpenoids | cryptotanshinone | Anticancer [140]. |
| Drought | S. miltiorrhiza [139] | rosmarinic acid | Decrease | Phenolics | rosmarinic acid | Antioxidant [141] |
| salvianolic acid | Increase | salvianolic acids | Antioxidant [142] | |||
| Drought | Scrophularia ningpoensis (Scrophulariaceae) [143] | catalpol, harpagide, aucubin, harpagoside | Increase | Glycosides | catalpol, aucubin | Hepatoprotective [144]; neuroprotective [145] |
| Ozone (O3) stress | S. lycopersicon [87,97] | α-carotene, β-carotene, violoxanthin | Increase | Terpenoids | β-carotene | Antioxidants [146]; anti-inflammatory [147] |
| isoprene, α-pinene, β-pinene, myrcene, limonene, sabinene, (E)-β-ocimene, (Z)-β-ocimene, α-humulene, (E)-β-farnesene, (E,E)-α-farnesene, (E)-β-caryophyllene, δ-cadinene | Increase | Terpenoids | α-pinene; myrcene; limonene; α-humulene. | Anti-inflammatory [148]; anti-asthmatic [149]; antioxidant [150]; anti-inflammatory [151] | ||
| O3 | Gingko biloba (Ginkgoaceae) [152] | ginkgolide A | Increase | Terpenoids | ginkgolide A | Neuroprotective [153] |
| Ultraviolet radiation-B (UV-B) | Arabidopsis thaliana (Brassicaceae) [154] | kaempferol 3-gentiobioside-7-rhamnoside; kaempferol 3,7-dirhamnoside. | Increase | Phenolics (Flavonoids) | NA | NA |
| UV-B | Brassica napus (Brassicaceae) [155] | quercetin 3-sophoroide-7-glucoside; quercetin 3-sinapyl sophoroside-7-glucoside | Increase | Phenolics (Flavonoids) | NA | NA |
| UV-B | Brassica oleracea (Brassicaceae) [156] | cyanidine glycosides; sinapyl alcohol | Increase | Phenolics (Flavoboids) | NA | NA |
| UV-B | C. roseus (Apocynaceae) [157,158] | catharanthine, vindoline | Increase | Alkaloids | catharanthine | Anticancer [159] |
| Clarkia breweri (Onagraceae) [160] | eugenol, isoeugenol, methyleugenol, and isomethyleugenol | Increase | Phenolics | eugenol | Antifungal [161]; anti-inflammatory [162] | |
| UV-B | Fagopyrum esculentum (Polygonaceae) [163] | rutin, quercetin, catechin | Increase | Phenolics | quercetin; catechin | Antioxidant [164]; anticancer and antioxidant [165,166] |
| UV-B | Gnaphalium luteoalbum (Asteraceae) [167] | calycopterin; 3’-methoxycalycopterin | Increase | Phenolics (Flavonoids) | calycopterin | Anticancer [168] |
| UV-B | G. viravira [169] | 7-O-methyl araneol | Increase | Phenolics (Flavonoids) | NA | NA |
| UV-B | Hordeum vulgare (Poaceae) [170] | saponarin; luteolin | Increase | Phenolics (Flavonoids) | saponarin; luteolin | Antihypertensive [171]; antibacterial [172] |
| UV-B | Marchantia polymorpha (Marchantiaceae) [173] | luteolin 7-glucuronide; luteolin 3,4’-di-p-coumaryl-quercetin 3-glucoside. | Increase | Phenolics (Flavonoids) | NA | NA |
| UV-B | Quercus ilex (Fagaceae) [174] | acylated kaempferol glycosides | Increase | Phenolics (Flavonoids) | kaempferol | Anticancer [175]; anti-inflammatory [176] |
| Heat stress | C. acuminata [177] | 10-hydroxycamptothecin | Increase | Alkaloids | 10-hydroxycamptothecin | Anticancer [178] |
| Heat stress | Daucus carota (Apiaceae) [179,180,181] | α-terpinolene | Decrease | Terpenoids | α-terpinolene | Antioxidant and anticancer [182] |
| α-caryophyllene, β-farnesene | Increase | NA | NA | |||
| anthocyanins, coumaric and caffeic acid; | Increase | Phenolics | p-coumaric acid and caffeic acid | Antioxidant [183,184] | ||
| Heat stress | Q. rubra (Fagaceae) [185] | isoprene (2-methyl-1,3-butadiene) | Increase | Terpenoids | NA | NA |
| Heat stress | S. lycopersicon [87,97] | β-phellandrene (dominant), 2-carene, α-phellandrene, limonene; increased emission of (E)-β-ocimene after treatment above 46 °C; β-caryophyllene. | Increase | Terpenoids | α-phellandrene; β-caryophyllene | Antifungal [186]; anticancer and anti-inflammatory [102,103] |
| α-humulene | Decrease | α-humulene | Anticancer [187] | |||
| Heat stress (increased humidity) | Centella asiatica (Apiaceae) [188] | asiaticoside | Increase | Phenolics | asiaticoside | Anti-cellulite agent [189] |
Abbreviations: NA: not available; LOX: lipoxygenase; UV: ultraviolet; ROS: reactive oxygen species.
3.1. Effects of Heat Stress on PSMs
Warming causes the accumulation of terpenoids, which usually have protective functions in mitigating environment-induced oxidative stress in plants [87,190]. For instance, tomato (S. lycopersicum) grown under heat stress (at 46 °C) emits higher levels of monoterpenes such as α-thujene, α-pinene, camphene, 2-carene, α-phellandrene, δ-3-carene (car-3-ene), α-terpinene, limonene, β-phellandrene, (E)-β-ocimene, and terpinolene; and also sesquiterpenes such as δ-elemene, β-elemene, α-humulene, and β-caryophyllene (Table 1) compared to controls [97]. In contrast, Nogués et al. [191] observed decreased emission of terpenes in Citrus monspeliensis grown under laboratory conditions at 35 °C; instead, increased assimilation of water-soluble antioxidant ascorbate indicates a shift from terpene-mediated to ascorbate-mediated ROS scavenging mechanism. Moreover, when C. monspeliensis was grown in the field, total terpene emission was higher during winter than in summer [191]. These contrasting findings suggest that terpene emissions under heat conditions could be species-specific and vary seasonally. Additionally, free fatty acids released by membrane phospholipase in response to heat (and cold) form lipoxygenase (LOX) products via lipoxygenase pathway, out of which C6 compounds (Z)-3-hexenal and (E)-2-hexenal are most common [97]. Wounded plants also release these two compounds within a few minutes [192,193]. Notably, (E)-2-hexenal acts as a chemical signal inducing the expression of stress-related transcription factors such as HSFA2 (heat stress transcription factor A-2) and MBF1c (multiprotein-bridging factor 1c) [194]. Heat stress may cause the melting of cuticular lipids, thus increasing cuticular permeability [195], and extreme temperatures may rupture terpene-containing-glandular trichomes, releasing the contents into the air [97]. After exposure to cold and heat stresses, favorable pH conditions inside plastids favor increased terpene synthesis [97] (Figure 5).
Under simulated environmental conditions, heat stress damages membranes (e.g., thylakoid membrane) and disintegrates membrane protein complexes (e.g., photosystem II) [196], consequently decreasing the rate of photosynthesis. Plants counteract such damage through sustained synthesis and emission of terpenes [87,197]. Korankye et al. [197] proposed that plants produce more terpenes under stressful conditions by diverting carbon to a non-mevalonate pathway, which otherwise could have been used in photosynthesis. Monoterpenes such as 1,8-cineole, α-terpinyl acetate, linalyl acetate, limonene, sabinene, myrcene, α-terpinen, β-ocimene, α-terpinolene, and γ-terpinene are most produced following decreased photosynthesis in plants [191,198]. Non-targeted PSMs profiling in tomatoes revealed higher concentrations of α-tocopherol and plastoquinone under 38 °C compared to lower temperatures (20 and 10 °C) [199]. Taken together with other studies [200,201], this suggests that these compounds function as electron carriers and facilitate photosynthesis in addition to their anti-oxidative functions. The photosynthetic rate also decreases under the increasing temperature as in Pueraria lobata [Willd.] Ohwi., and Quercus spp. when isoprene synthesis (non-mevalonate pathway) was inhibited with fosmidomycin [202]. They suggest that isoprene improves thermotolerance in plants and helps photosynthetic apparatus recover after experiencing heat shock (i.e., temperature > 40 °C). Studies [203,204] suggest that plants tolerant to sunlight-induced heat flecks, O3, and ROS produce more isoprene than non-tolerant species. However, not all plants seem to produce isoprenoid compounds, but it varies among different plant species. For instance, when grown at 30 °C, Salix phylicifolia L. emitted isoprene, whereas Betula nana L. and Cassiope tetragona (L) D.Don emitted monoterpenes such as (Z)-2-hexenal, hexenyl butyrate, hexenyl acetate, and 3-hexenyl-methyl butanoate [205]. Heat stress also enhances the production of water-soluble antioxidants (e.g., ascorbate and glutathione) as well as lipid-soluble antioxidants (e.g., tocopherols) that scavenge increasing ROS [206,207]. For example, Lycopersicon esculentum Mill. Var. Amalia, after receiving heat shock at 45 °C for three hours, has been shown to produce more ascorbate and glutathione than its wild thermotolerant type Nagcarlang control under the same conditions [207]. Heat stress also affects flavonoids production as sweet basil (Ocimum basilicum L.) responds to high temperatures by producing flavonoids [208].
3.2. Effects of Cold Stress on PSMs
Cold stress or low-temperature stress is either chilling (<20 °C) or freezing (<0 °C) temperature, and they adversely affect plants’ growth and development. Plants growing in sub-tropical and tropical areas are more sensitive to cold stress than temperate species [209]. Cold stress tolerance in plants is achieved through selective expression of stress-defensive genes, which is reviewed by Chinnusamy et al. [210]. For instance, Jeon et al. [106] investigated transcripts and metabolites in six-day-old tartary buckwheat (Fagopyrum tartaricum) after cold exposure (at 4 °C, for various periods), observing upregulation of phenylpropanoid biosynthetic transcripts and significant accumulation of anthocyanins and proanthocyanidins, both antioxidative (Table 1) [107]. When two varieties of grapevine Vitis vinifera L. (cold tolerant – Maerchal Foch, and cold-sensitive – Kiszmisz Luczistyj) were exposed to 10/7 °C day/night cycle for 14 h photoperiod at 180–200 μm/(m2s) irradiance, the cold-tolerant variety had higher total phenolic compound content when assessed using the Folin-Ciocalteu’s reagent [211]. Subsequently, when they tested the antioxidant capacities of leaf extracts from two varieties by DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging assay, leaves from cold-tolerant varieties yielded better activity.
Another exciting example of the role of PSMs in plants under cold stress is the medicinal plant, Indian ginseng (Withania somnifera L.), which is the primary source of biologically active withanolides. Mir et al. [108] studied the accumulation of withanolides in response to cold stress in two genotypes of W. somnifera (AGB002―wild genotype and AGB025―cultivated genotype). After subjecting these two genotypes to chilling temperature (4 °C, for a maximum of seven days), bioactive compounds such as withanolide A in the roots and withaferin A in leaves were detected in both genotypes, suggesting the involvement of withanolides in cold tolerance. Moreover, the wild genotype showed a higher accumulation of marker withanolides than the cultivated one, which could mean that plants may not produce relevant bioactive compounds when out of their natural habitat, which is discussed later.
Glucosylated terpenoids (e.g., some sesquiterpenes) are another group of PSM involved in cold stress tolerance. Zhao et al. [111] reported the accumulation of glucosylated sesquiterpene and nerolidol glucoside (i.e., catalyzed by plant glycosyltransferase, UGT91Q2) in tea plants (Camellia sinensis) in response to cold stress (freezing temperature, −5 °C, for 4 h). The accumulation of nerolidol glucoside was directly proportional to the expression level of UGT91Q2, indicating that cold stress induces glycosylation in tea. Moreover, the ROS-scavenging ability of nerolidol glucoside was significantly higher than nerolidol, thus increasing cold tolerance in tea.
3.3. Effects of Drought Stress on PSMs
Climate change is expected to alter precipitation patterns and results in drought stress (water deficit) in some plants. Drought stress is considered major abiotic stress that impedes metabolism [212,213] and leads to changes in plants at the morphological, physiological, biochemical, metabolic, and transcriptional levels. ROS formation is one drought stress effect, which damages cellular components, including proteins, lipids, and nucleic acids [214,215]. Accumulation of flavonoids such as flavonols and anthocyanins is essential in protecting against abiotic stresses, including drought stress, but the mechanism of action is poorly understood [216]. For example, concentrations of antioxidant flavonols epigallocatechin gallate, epicatechin, and epicatechin gallate increase in the leaves of Cistus clusii under drought stress, reaching a maximum after 30 days of exposure [120,217]. However, the efficacy of photosystem II (PSII) and lipid peroxidation remained unchanged. Under drought stress, PSII in the cotton (Gossypium hirsutum) also remained unaffected [218]. Nakabayashi et al. [216,219] also obtained a similar result (increasing flavonols and anthocyanins) under drought stress in the aerial parts of Arabidopsis thaliana (wild type, Col-0) and confirmed that overaccumulation of flavonoids is key to drought tolerance. There was also a drastic increase in the concentrations of glycosides of kaempferol, quercetin, and cyanidin along with drought stress marker metabolites (proline, raffinose, and galactinol). Excessive accumulation of anthocyanins protects plants against drought stress [219], and anthocyanins are thought to be more robust antioxidants due to their higher level of hydroxylation [220]. A few other studies [221,222] have reported similar observations, i.e., increased accumulation of anthocyanins in plants under drought. Drought stress in Amaranthus tricolor genotype VA3 increased concentrations of at least 16 phenolic compounds, including six hydroxybenzoic acids, seven hydroxycinnamic acids, three flavonoids, and a new phenolic acid, trans-cinnamic acid (Table 1) [112]. In tea plants, fulvic acid is the primary driver of tolerance against drought stress by enhancing ascorbate and glutathione metabolism and promoting flavonoids biosynthesis [223]. More examples and patterns of biochemical changes induced by drought stress in plants are given in Table 1.
3.4. Effects of Ultraviolet (UV) Radiation on PSMs
Plants respond to excessive ultraviolet radiation (UV) both morphologically and physiologically. UV radiation is known to trigger a wide range of responses in plant cells, mainly by UV-B (280–320 nm) and less by UV-A (315–400 nm). Plants’ response to UV stress depends on their perception, signal transduction mechanism, and influence of gene expression [224]. Other environmental factors also influence response to UV-B stress in plants as UV radiation indirectly damages the photosynthetic apparatus by generating ROS [225]. Thus, plants have developed a mechanism to protect against UV radiation and allow photosynthetically active radiation (PAR) to reach mesophyll and palisade tissues in order to enable photosynthesis. Synthesizing UV-absorbing flavonoids is one mechanism to mitigate photoinhibition and photooxidative damage by either reducing UV penetration or quenching ROS. Flavonoids can absorb radiation in the UV region of the spectrum; thus, these compounds are responsible for filtering UV light in plants [226]. Unlike other lights of different wavelengths, UV-B radiation can damage DNA and chloroplasts, particularly photosystem II (PSII) and modify or inhibit gene expression due to its high energy, and they are absorbed by a wide range of molecules [227]. When Stapleton and Walbot [226] investigated DNA damage in maize plants exposed to UV-C or UV-B radiation at a dose of 6000 J/m2, maize plants with flavonoids, primarily anthocyanins, suffered less DNA damage than maize plants deficient in flavonoids. Flavonoids with a catechol group in their B-ring skeleton (e.g., quercetin derivatives) are best known to protect photosynthetic tissues from such oxidative damage [228]. Moreover, exposure to excess UV-B radiation causes increased synthesis of stronger antioxidants such as dihydroxy B-ring-substituted flavonoids (e.g., quercetin and luteolin glycosides) (Figure 5) and less effective antioxidant flavonoids such as kaempferol or apigenin glycosides [229,230]. As a response to UV irradiation, the concentrations of quercetin flavonoids increase in Brassica napus [156] and Fagopyrum esculentum [163]. The concentration of antioxidative flavonoids increased in Kalanchoe pinnata when exposed to UV-B radiation compared to ordinary white light [231]. When Del Valle et al. [225] investigated the effects of UV radiation in Silene littorea, UV exposure increased the concentrations of protective phenolic compounds but affected its reproductive efficacy. UV-B radiation modifies gene expression, but their underlying molecular mechanism is not well understood, unlike other phytochrome and blue/or UV-A. Herrlich et al. [232] attribute plant response to UV-B stress mainly to damage caused to cell membranes and DNA. The multiple roles of flavonoids, including photoprotection and the effects of stress on flavonoid biosynthesis, are reviewed elsewhere [52,54].
3.5. Effects of Ozone on PSMs
Ozone (O3) in the lower atmosphere (troposphere) acts as a greenhouse gas and is phototoxic to plants [233]. It is usually produced by reactions between primary pollutants (such as carbon oxides, sulphur oxides, nitric oxides, and hydrocarbons) catalyzed by sunlight. Although O3 is neither a free radical nor a ROS, its strong oxidizing properties enable it to react with biomacromolecules, including lipids, proteins, nucleic acids, and carbohydrates [234]. Generally, O3 enters through stomata and damages leaf tissues, mainly in the upper (adaxial) layers resulting in chlorosis and lesions. Physiologically, exposure to O3 impairs stomatal function (dysfunction of transpiration and water use efficiency) and reproductive development, CO2 assimilation, and subsequently photosynthetic activity. In snap bean (Phaseolus vulgaris), exposure to an ambient concentration of O3 (≤150 ppb, 1 h) [along with water stress (≤15%)] induces sluggishness in stomatal closure, subsequently causing more significant loss of leaf surface water [235].
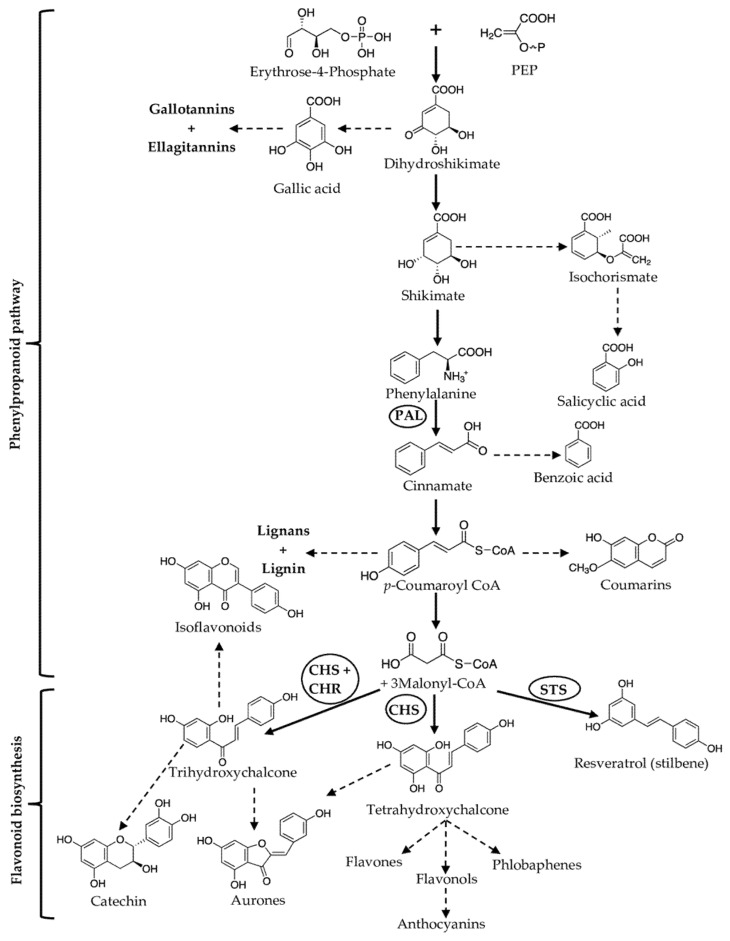
In addition to changes in plant physiological functions, O3 triggers pathways responsible for producing defensive molecules, such as flavonoids. When Mao et al. exposed soybean leaves to elevated O3 (110 ± 10 nmol mol−1 for 8 h daily, for 54 days), the concentrations of rutin, quercetin, and total flavonoids increased significantly [236]. Ozone also enhances the activity of enzymes involved in flavonoid biosynthesis. Plants fumigated with O3 show increased activities of phenylalanine-ammonium lyase (PAL), and chalcone synthase (CHS) enzymes involved in phenylpropanoid and flavonoid biosynthesis pathways [237] and subsequently produce protective compounds that can scavenge ROS [56]. The general phenylpropanoid pathway and flavonoid biosynthesis pathways are outlined in Figure 6 below. These pathways, in turn, contribute significantly towards plant defense response by producing protective phenolic compounds such as condensed tannins and flavonoids that can scavenge ROS [57]. For instance, when Arabidopsis thaliana is exposed to O3 (300 ppb daily for 6 h), PAL mRNA levels increase 3-fold compared to their control plants [238]. Similarly, O3 treatment (200 nL/L for 10 h) increases both PAL and CHS activities resulting in a 2-fold increase of total leaf furanocoumarins and flavone glycosides in parsley (Petroselinum crispum) [239]. Lignin deposition in O3 exposed leaves is also linked to increased PAL activity [240], whereas in sage (Salvia officinalis), both PAL and PPO (phenol oxidase) activities were suppressed after 24 h exposure to O3 [241]. However, rosmarinic acid synthase (RAS) activity is accompanied by the increased transcription level of genes (e.g., RAS) encoding biosynthesis enzymes, suggesting that the sage plant mediates oxidative damage through synthesizing phenolic compounds.
Figure 6.
General phenylpropanoid pathway and flavonoid biosynthesis (adapted from [247,248]. Solid arrows represent single enzymatic reaction; dashed arrows represent multiple sequential reactions. Enzymes involved: PAL—phenylalanine ammonia lyase; CHS—chalcone synthase; STS—stilbene synthase; CHR—chalcone reductase.
Studies have shown that plant chemical responses to O3 exposure variably depend on the O3 concentration [242]. Ozone alone enhances the production of phenolic compounds more significantly than in response to the increased CO2 concentration, while the combination of these two factors resulted in higher diterpenes, but not mono- and sesquiterpene, synthesis in plants [243]. However, some experiments showed contrasting results from O3 fumigation. Leaves of Ginkgo biloba, upon fumigation with an elevated level of O3, increased the concentrations of terpenes (Table 1), but phenolics decreased [152]. Ozone also enhances the accumulation of salicylic acid (SA) in plant tissues; for instance, in the tobacco plant (Nicotiana tabacum), emission of SA-derived methyl salicylate increases upon exposure to O3 [244,245]. In Arabidopsis, SA accumulation is necessary for forming O3-induced mRNAs, such as PAL and pathogenesis-related protein 1 (PAR1) transcripts [245]. Nevertheless, some plants (such as tobacco plants) do not require SA accumulation to form PAL transcripts [246]. These examples suggest that O3 induces at least two signaling pathways, the SA-dependent pathway associated with pathogen defense response and the SA-independent pathway in the protective response to O3.
Isoprene in tree foliage is known to protect foliage from oxidative stress. For instance, when Loreto et al. [204] applied isoprene (2–3 ppm) exogenously to tobacco and birch leaves fumigated with O3 (300 ppb), photosynthesis was consistent throughout the treatment period with the less accumulation of ROS compared to their fosmidomycin-treated control (showed more ROS accumulation and decreased rate of photosynthesis). Moreover, after three days of O3 treatment, they observed that areas of leaves treated with isoprene were intact, suggesting that isoprene protects photosynthetic tissues and stabilizes the thylakoid membrane. Isoprene protects photosynthesis in those plants exposed to acute thermal and O3 stress through antioxidative action (quench H2O2) and preventing membrane lipid peroxidation. For instance, leaves of Phragmites australis for which their endogenous isoprene production was inhibited by applying fosmidomycin become more sensitive to O3 stress than isoprene-producing leaves [204].
Exposure to high O3 concentration causes VOC emission, but at chronic O3 level, it modifies compositions of BVOCs, consequently affecting tri-trophic interactions and weakening plants’ response to arthropod attack [245,249]. During such situations, isoprenoids (mainly hemiterpenes, monoterpenes, and sesquiterpenes) are synthesized by plants to tolerate O3-induced damages. Hemiterpene is an example of an isoprenoid released in the leaves, as it can protect photosynthetic apparatus and scavenge O3 by-products and ROS due to its antioxidative activity [204]. The effect of O3 on alkaloid biosynthesis remains less elucidated, but polyamines in plants, which is an important alkaloid precursor, are correlated to O3 tolerance [234]. Polyamines in plants possess a wide array of physiological functions [240] in addition their involvement in response to both abiotic and biotic stresses [250].
4. Reported Pharmacological Properties of PSMs Present in Plants Affected by Ex Situ Abiotic Stresses
Plant protective secondary metabolites are diverse in structure and biological properties, and they have been continuously exploited for pharmaceutical, nutraceutical, and cosmetic uses [251] (Figure 7). Flavonoids and other phenolic compounds are predominant among secondary metabolites produced in response to climatic/or abiotic stress (Table 1). Flavonoids confer protection against inflammation, allergy, and bacterial infections [252]. Flavonols (or 3-hydroxy flavones), one of the main subclass of flavonoids, are apparent antioxidants in stressed plants, and they are known to prevent nuclear DNA damage by free radicals like H2O2 [253]. Flavonols are polyaromatic secondary metabolites with three rings, and many of them are bioactive. Many flavonoids possess antiviral properties. For instance, the hydroxy (OH) group in the ring-C of flavonols makes them more effective against herpes simplex virus type I than flavones [254]. Fisetin is another example of an active flavonoid produced by plants under oxidative stress, preventing membrane lipid peroxidation, DNA damage, and protein carbonylation [247]. Fisetin showed numerous biological activities such as protection against cell death from oxidative stress, growth, and maintenance of nerve cells (primary cortical neurons from a rat) [248,255]. Fisetin suppresses many inflammatory pathways, including Nuclear Factor-kappa B (NF-kB) pathway, helping prevent cancerous growth [256,257]. Similarly, Hussain et al. [258] also observed the protective effect of fisetin against smoke-induced oxidative stress and inflammation in rat lungs. Plant UV filters, kaempferol, and quercetin are a few other examples of bioactive flavonoids. Kaempferol is an anti-inflammatory [259], chemo-protective [260], and cardio-protective [261]. Polyphenolic resveratrol is one of the essential stilbene phytoalexin produced by a plant’s defense mechanism, and it possesses antioxidant, anticancer, and anti-estrogenic properties [262]. The immunoinhibitory compound, calycopterin isolated from the medicinal plant Dracocephalum kotschyi [168], was elevated upon UV irradiation in Gnaphalium luteo-album [167]. Tanshinones are other examples of bioactive phenols. In response to severe drought stress, their concentration in the Salvia miltiorrhiza increases, including tanshinone I and tanshinone IIA by 182% and 322%, respectively, compared to 148% under the moderate drought stress [139]. Tanshinones are known for their anti-inflammatory, antioxidant, and anticancer properties [263].
Figure 7.
Chemical structure of compounds known to accumulate in plants under various abiotic stress conditions.
Nitrogen-containing compounds, alkaloids, are another group of secondary metabolites widely produced in plants for defense, and they are known to exhibit diverse biological activities, including anti-inflammatory, anti-malarial, and anticancer activities [264]. The fungistatic activity of α-tomatine (Solanum and Lycopersicon species) in Fusarium oxysporum f. lycopersici (tomato wilt) was the first bioactive alkaloid reported in 1945 by Irving et al. [69]. Alkaloids and their precursors accumulate more in plants when exposed to various stress factors. For example, Catharanthus roseus, when exposed to UV-B radiation, synthesizes more indole alkaloids and precursors of vinblastine and vincristine increase in hairy roots [265]. These alkaloids inhibit cell mitosis by destroying microtubules of the mitotic apparatus, blocking cancer cell division [266]. Bioactive alkaloids accumulate in response to high temperature, drought, and UV-B stresses (Table 1). Indole alkaloid vindoline from Catharanthus roseus (which increases in response to UV-B) showed anti-diabetic (reduces fasting blood glucose level) and anti-inflammatory (reduces pro-inflammatory cytokines, TNF- α and IL-6) properties [99].
The number of structurally determined specialized plant terpenes exceeds 105, including >12,000 diterpenoids [267]. Plant terpenoids are diverse and have been a valuable source of medicinal discoveries because terpenoids are natural NF-kB signaling inhibitors with anti-inflammatory and anti-cancer properties [268]. Examples include monoterpenes (e.g., (−)-menthol and cannabinoids); sesquiterpenes (e.g., artemisinin and thapsigargin); diterpenes (e.g., paclitaxel and ingenol mebutate) and triterpenes found in floral and vegetative parts; triterpenoids; and carotenoids (e.g., steroidal alkaloids, cardenolides, and bixin) (Figure 7). Other compounds are partially derived from a terpene precursor, such as monoterpenoid alkaloids (e.g., strychnine), which are synthesized in part from secologanin (Figure 7), a member of the widespread class of iridoid monoterpenes [269].
5. Biodiscovery Potential of Plants Growing under Ex-Situ Abiotic Stresses
Natural products, including PSMs, have been a significant source of medicines. According to Newman and Cragg, between 1981 and 2010, 1073 small molecules (mol. wt. < 1000 Da) were approved as new chemical entities, out of which more than half were from natural products [270]. An additional 321 small molecules were reported in another review published in September 2019 [271]. According to Butler et al. [272], in their review covering natural products-derived drugs between 2008–2013, 25 drugs were launched since 2008, and additional 31 compounds were in the last stage clinical trial (phase III). According to the database on www.clinicaltrials.gov (accessed 5 September 2021), four compounds have advanced to phase-IV clinical trial, sixteen have completed phase-III, nine have not yet completed phase-III, and two compounds have been withdrawn. The four compounds that have advanced to clinical trial phase-IV are oritavancin (anti-bacterial), ipragliflozin, tofoglifozin (anti-diabetic, type II diabetes), and vorapaxar (anti-thrombotic) [272]. Recently, pharmaceutical industries and researchers have renewed their interest in PSMs due to advancements in cutting-edge technology, including various chromatography and high-resolution spectroscopy tools and omics platforms [273].
Interestingly, not many PSMs were subjected to clinical trials. The reasons are varied. One of the continuing challenges for drug discovery from plant sources is obtaining enough sample extracts and compounds for testing in vitro and in vivo disease models. This bottleneck is heightened for species in the IUCN red list of threatened or endangered species prohibited for large sample collection, even if they show biological hits. While cultivating pharmaceutically interesting plant species may be a solution, it is not always possible to culture the organism outside its natural habitat. Even when possible, relevant natural products may not be produced outside their natural habitat [273]. Alternatively, plants affected by climate change could be a potential source of novel drug leads, considering the vast diversity of phytochemicals produced by them in response to various abiotic stress conditions (Table 1).
Climate change rapidly and severely affects plant ecosystems; for instance, mountaintop ecosystems are sensitive to small shifts in temperature and precipitation patterns [274]. Several studies on the mountaintops of the Asia-Pacific region [275], Oceania [276], and Europe [277] have reported accelerated plant ecological responses, including distribution, ecophysiology, and interaction with other organisms due to climatic changes. In overcoming climate change-induced/or abiotic stress and finding an optimal climate niche, plants produce diverse PSMs, which could be of pharmaceutical interest. For example, the synthesis of plant terpenoids increases under heat, cold, and O3 stress, and the yield of many biologically active compounds also increases in plants grown in simulated environments of various abiotic stress conditions (Table 1). Abiotic stresses elicit bioactive compound synthesis [278], such as phenylpropanoids biosynthesis (mainly through shikimate pathway), causing an accumulation of compounds with defense or signaling functions (e.g., phenolics, flavonoids, and alkaloids) [279]. Similarly, it is reported that drought stress increases the concentration of camptothecin (anticancer alkaloid) in Camptotheca acuminata [116,117] and morphine (analgesic) concentrations in Papaver somniferum. The increased accumulation of PSMs in response to stress indicates that there may be novel bioactive alkaloid(s) in climate change-affected plants awaiting discovery. Abiotic stress factors under conditioned environment can potentially improve the yield of bioactive compounds in plants.
6. Conclusions
Plants constantly interact with the environment, and climate change has already impacted their diversity, growth, and survival. In order to minimize the impact of various climate change-related stresses (such as warming due to increased greenhouse gas emission, drought, cold, ozone-layer depletion, and harmful UV-radiation), plants produce diverse defense secondary metabolites, mainly phenolic and nitrogen-containing compounds. The biosynthesis of defense compounds in plants (including medicinal plants) is often upregulated, and these compounds are associated with various pharmacological properties, suggesting that plants affected by climate change may be a rich resource for drug discovery. However, most of these studies were conducted in simulated/or artificial environments. Thus, it would be interesting if more such studies (defense compounds produced by plants in response to climatic stress and their bioactivity) could be conducted by using plant samples from their natural habitats that are already challenged by the various climatic stresses.
It is difficult to access various natural products bound by legislation and societal restrictions, including plants, for drug discovery research, particularly plants associated with indigenous knowledge. This limitation remains a considerable challenge for those working with medicinal plants. Other wild plants exposed to various climatic/or abiotic stresses would be an alternative option for drug discovery researchers. Another obstacle in the drug discovery process is obtaining adequate compounds for further biological tests (both in vitro and in vivo). Bioactive compounds increase their concentration in plants exposed to stress, for example, withanolides in Indian ginseng (Withania somnifera) increases in response to cold stress. Culturing plant tissues of interest at a large scale under a conditioned environment using various abiotic stresses can potentially improve the yield of bioactive compounds from plants. Thus, plant tissue culture would be another platform for researchers and pharmaceutical industries to upscale the production of valuable phytochemicals under duress of climate change factors.
Author Contributions
Conceptualization, P.W. and K.Y.; writing—original draft preparation, K.Y.; writing—review and editing, P.W., D.C. and E.R.; supervision, P.W.; funding acquisition, P.W. and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This review was funded by James Cook University Postgraduate Research Scholarship (JCUPRS) to Karma Yeshi; NHMRC Ideas grant (APP1183323) to Phurpa Wangchuk and Darren Crayn; and research grants from the Ian Potter Foundation and the Wet Tropics Management Authority to Darren Crayn.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kessler A., Kalske A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 2018;49:115–138. doi: 10.1146/annurev-ecolsys-110617-062406. [DOI] [Google Scholar]
- 2.Sallas L., Luomala E.M., Utriainen J., Kainulainen P., Holopainen J.K. Contrasting effects of elevated carbon dioxide concentration and temperature on rubisco activity, chlorophyll fluorescence, needle ultrastructure and secondary metabolites in conifer seedlings. Tree Physiol. 2003;23:97–108. doi: 10.1093/treephys/23.2.97. [DOI] [PubMed] [Google Scholar]
- 3.Julkunen-Tiitto R., Nenadis N., Neugart S., Robson M., Agati G., Vepsäläinen J., Zipoli G., Nybakken L., Winkler B., Jansen M.A.K. Assessing the response of plant flavonoids to UV radiation: An overview of appropriate techniques. Phytochem. Rev. 2014;14:273–297. doi: 10.1007/s11101-014-9362-4. [DOI] [Google Scholar]
- 4.Kaling M., Kanawati B., Ghirardo A., Albert A., Winkler J.B., Heller W., Barta C., Loreto F., Schmitt-Kopplin P., Schnitzler J.P. UV-B mediated metabolic rearrangements in poplar revealed by non-targeted metabolomics. Plant. Cell Environ. 2015;38:892–904. doi: 10.1111/pce.12348. [DOI] [PubMed] [Google Scholar]
- 5.Hošek J., Šmejkal K. Flavonoids as anti-inflammatory agents. In: Miller R., editor. Encyclopedia of Inflammatory Diseases. Hayle Medical; New York, NY, USA: 2015. pp. 1–17. [DOI] [Google Scholar]
- 6.Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines. 2018;5:93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeshi K., Yangdon P., Kashyap S., Wangchuk P. Antioxidant activity and the polyphenolic and flavonoid contents of five high altitude medicinal plants used in Bhutanese sowa rigpa medicine. JBAPN. 2017;7:18–26. doi: 10.1080/22311866.2017.1287593. [DOI] [Google Scholar]
- 8.Pinto D.M., Blande J.D., Souza S.R., Nerg A.M., Holopainen J.K. Plant volatile organic compounds (VOCs) in ozone (O3) polluted atmospheres: The ecological effects. J. Chem. Ecol. 2010;36:22–34. doi: 10.1007/s10886-009-9732-3. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton A.C. Medicinal plants, conservation and livelihoods. Biodivers. Conserv. 2004;13:1477–1517. doi: 10.1023/B:BIOC.0000021333.23413.42. [DOI] [Google Scholar]
- 10.Applequist W.L., Brinckmann J.A., Cunningham A.B., Hart R.E., Heinrich M., Katerere D.R., van Andel T. Scientists’ warning on climate change and medicinal plants. Planta Med. 2020;86:10–18. doi: 10.1055/a-1041-3406. [DOI] [PubMed] [Google Scholar]
- 11.Roy S.K., Roy D.K. Use of medicinal plant and its vulnerability due to climate change in northern part of Bangladesh. Am. J. Plant. Sci. 2016;07:1782–1793. doi: 10.4236/ajps.2016.713166. [DOI] [Google Scholar]
- 12.Gupta A., Singh P.P., Singh P., Singh K., Singh A.V., Singh S.K., Kumar A. Climate Change and Agricultural Ecosystems. Elsevier; Amsterdam, The Netherlands: 2019. Medicinal Plants Under Climate Change: Impacts on Pharmaceutical Properties of Plants; pp. 181–209. [Google Scholar]
- 13.Trouvelot S., Heloir M.C., Poinssot B., Gauthier A., Paris F., Guillier C., Combier M., Trda L., Daire X., Adrian M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant. Sci. 2014;5:1–14. doi: 10.3389/fpls.2014.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S., Choi H., Suh S., Doo I.S., Oh K.Y., Choi E.J., Taylor A.S., Low P.S., Lee Y. Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 1999;121:147–152. doi: 10.1104/pp.121.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albert M. Peptides as triggers of plant defence. J. Exp. Bot. 2013;64:5269–5279. doi: 10.1093/jxb/ert275. [DOI] [PubMed] [Google Scholar]
- 16.Marmiroli N., Maestri E. Plant peptides in defense and signaling. Peptides. 2014;56:30–44. doi: 10.1016/j.peptides.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Isah T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019;52:1–25. doi: 10.1186/s40659-019-0246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kesselmeier J., Staudt M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999;33:23–88. doi: 10.1023/A:1006127516791. [DOI] [Google Scholar]
- 19.Neilson E.H., Goodger J.Q.D., Woodrow I.E., Møller B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013;18:250–258. doi: 10.1016/j.tplants.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Willis K.J. State of the World’s Plants 2017. [(accessed on 9 November 2021)]. Available online: https://www.kew.org/about-us/press-media/state-of-the-worlds-plants-2017.
- 21.van Wyk B.E. The value of chemosystematics in clarifying relationships in the genistoid tribes of papilionoid legumes. Biochem. Syst. Ecol. 2003;31:875–884. doi: 10.1016/S0305-1978(03)00083-8. [DOI] [Google Scholar]
- 22.Acamovic T., Brooker J.D. Biochemistry of plant secondary metabolites and their effects in animals. Proc. Nutr. Soc. 2005;64:403–412. doi: 10.1079/PNS2005449. [DOI] [PubMed] [Google Scholar]
- 23.Ritmejeryte E., Boughton B.A., Bayly M.J., Miller R.E. Unique and highly specific cyanogenic glycoside localization in stigmatic cells and pollen in the genus Lomatia (Proteaceae) Ann. Bot. 2020;126:387–400. doi: 10.1093/aob/mcaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sampaio B.L., Edrada-Ebel R., Da Costa F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep29265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryde I., Li T., Rieksta J., Dos Santos B.M., Neilson E.H.J., Gericke O., Jepsen J.U., Bork L.R.H., Holm H.S., Rinnan R. Seasonal and elevational variability in the induction of specialized compounds from mountain birch (Betula pubescens var. pumila) by winter moth larvae (Operophtera brumata) Tree Physiol. 2021;41:1019–1033. doi: 10.1093/treephys/tpab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grainger T.N., Gilbert B. Multi-scale responses to warming in an experimental insect metacommunity. Glob. Chang. Biol. 2017;23:5151–5163. doi: 10.1111/gcb.13777. [DOI] [PubMed] [Google Scholar]
- 27.Jogawat A., Yadav B., Chhaya, Lakra N., Singh A.K., Narayan O.P. Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: A review. Physiol. Plant. 2021;172:1106–1132. doi: 10.1111/ppl.13328. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Kong D., Fu Y., Sussman M.R., Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant. Physiol. Biochem. 2020;148:80–89. doi: 10.1016/j.plaphy.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Yadav B., Jogawat A., Rahman M.S., Narayan O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Reports. 2021;23:1–14. doi: 10.1016/j.genrep.2021.101040. [DOI] [PubMed] [Google Scholar]
- 30.Jamwal K., Bhattacharya S., Puri S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J. Appl. Res. Med. Aromat. Plants. 2018;9:26–38. doi: 10.1016/j.jarmap.2017.12.003. [DOI] [Google Scholar]
- 31.Aharoni A., Jongsma M.A., Bouwmeester H.J. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 2005;10:594–602. doi: 10.1016/j.tplants.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Song M.C., Kim E.J., Kim E., Rathwell K., Nam S.J., Yoon Y.J. Microbial biosynthesis of medicinally important plant secondary metabolites. Nat. Prod. Rep. 2014;31:1497–1509. doi: 10.1039/C4NP00057A. [DOI] [PubMed] [Google Scholar]
- 33.Breitmaier E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones. Wiley-VCH; Weinheim, Germany: 2006. pp. 1–223. [Google Scholar]
- 34.Pichersky E., Gershenzon J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant. Biol. 2002;5:237–243. doi: 10.1016/S1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- 35.Kessler A., Baldwin I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 36.Singh B., Sharma R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. Three Biotech. 2015;5:129–151. doi: 10.1007/s13205-014-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Lamothe R., Mitchell G., Gattuso M., Diarra M.S., Malouin F., Bouarab K. Plant antimicrobial agents and their effects on plant and human pathogens. Int. J. Mol. Sci. 2009;10:3400–3419. doi: 10.3390/ijms10083400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loreto F., Schnitzler J.P. Abiotic stresses and induced BVOCs. Trends Plant. Sci. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Dahham S.S., Tabana Y.M., Iqbal M.A., Ahamed M.B.K., Ezzat M.O., Majid A.S.A., Majid A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-Caryophyllene from the essential oil of Aquilaria crassna. Molecules. 2015;20:11808–11829. doi: 10.3390/molecules200711808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porres-Martinez M., Gonzalez-Burgos E., Carretero M.E., Gomez-Serranillos M.P. In vitro neuroprotective potential of the monoterpenes alpha-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z. Nat. C J. Biosci. 2016;71:191–199. doi: 10.1515/znc-2014-4135. [DOI] [PubMed] [Google Scholar]
- 41.Blanch J.S., Peñuelas J., Sardans J., Llusià J. Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta. Physiol. Plant. 2009;31:207–218. doi: 10.1007/s11738-008-0221-z. [DOI] [Google Scholar]
- 42.Bartwal A., Mall R., Lohani P., Guru S.K., Arora S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J. Plant. Growth Regul. 2013;32:216–232. doi: 10.1007/s00344-012-9272-x. [DOI] [Google Scholar]
- 43.Kozukue N., Han J.-S., Lee K.-R., Friedman M. Dehydrotomatine and alpha-tomatine content in tomato fruits and vegetative plant tissues. J. Agric. Food Chem. 2004;52:2079–2083. doi: 10.1021/jf0306845. [DOI] [PubMed] [Google Scholar]
- 44.Trojanowska M.R., Threlfall D.R. Regulation of saponin biosynthesis in primary roots of oat. Acta. Bot. Gall. 1999;146:101–104. doi: 10.1080/12538078.1999.10515805. [DOI] [Google Scholar]
- 45.Moses T., Papadopoulou K.K., Osbourn A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014;49:439–462. doi: 10.3109/10409238.2014.953628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman M. Potato glycoalkaloids and metabolites: Roles in the plants and in the diet. J. Agric. Food Chem. 2006;54:8655–8681. doi: 10.1021/jf061471t. [DOI] [PubMed] [Google Scholar]
- 47.Ndamba J., Lemmich E., Mølgaard P. Investigation of the diurnal, ontogenetic and seasonal variation in the molluscicidal saponin content of Phytolacca dodecandra aqueous berry extracts. Phytochemistry. 1994;35:95–99. doi: 10.1016/S0031-9422(00)90515-6. [DOI] [PubMed] [Google Scholar]
- 48.Li J.T., Chen S.L., Liu S.C., Yang D.J. Effect of harvest time on saponins in Yam (Dioscorea pseudojaponica Yamamoto) J. Food Drug Anal. 2009;17:116–122. [Google Scholar]
- 49.Hayat M., Abbas M., Munir F. Potential of plant flavonoids in pharmaceutics and nutraceutics. J. Biomol. Biochem. 2017;1:12–17. [Google Scholar]
- 50.Cheynier V., Comte G., Davies K.M., Lattanzio V., Martens S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant. Physiol. Biochem. 2013;72:1–20. doi: 10.1016/j.plaphy.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Hussein R.A., El-Anssary A.A. Herbal Medicine. Intechopen; London, UK: 2019. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants; pp. 11–30. [DOI] [Google Scholar]
- 52.Winkel-Shirley B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant. Biol. 2002;5:218–223. doi: 10.1016/S1369-5266(02)00256-X. [DOI] [PubMed] [Google Scholar]
- 53.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: An overview. J. Nutr. Sci. 2016;5:1–5. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agati G., Tattini M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010;186:786–793. doi: 10.1111/j.1469-8137.2010.03269.x. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi A., Ohnishi T. The significance of the study about the biological effects of solar ultraviolet radiation using the exposed facility on the international space station. Biol. Sci. Space. 2004;18:255–260. doi: 10.2187/bss.18.255. [DOI] [PubMed] [Google Scholar]
- 56.Harborne J.B., W’illiams C.A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/S0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 57.Samanta A., Das G., Das S. Roles of flavonoids in plants. Int. J. Pharm. Sci. Tech. 2011;6:12–35. [Google Scholar]
- 58.Schulz E., Tohge T., Zuther E., Fernie A.R., Hincha D.K. Flavonoids are determinants of freezing tolerance and cold acclimation in Arabidopsis thaliana. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep34027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pastore C., Dal Santo S., Zenoni S., Movahed N., Allegro G., Valentini G., Filippetti I., Tornielli G.B. Whole plant temperature manipulation affects flavonoid metabolism and the transcriptome of grapevine berries. Front. Plant. Sci. 2017;8:1–16. doi: 10.3389/fpls.2017.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bieza K., Lois r. An Arabidopsis mutant tolerant to lethal ultraviolet-B levels shows constitutively elevated accumulation of flavonoids and other phenolics. Plant. Physiol. 2001;126:1105–1115. doi: 10.1104/pp.126.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kabtni S., Sdouga D., Bettaib Rebey I., Save M., Trifi-Farah N., Fauconnier M.L., Marghali S. Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Sci. Rep. 2020;10:1–15. doi: 10.1038/s41598-020-65160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tattini M., Landi M., Brunetti C., Giordano C., Remorini D., Gould K.S.e.a. Epidermal coumaroyl anthocyanins protect sweet basil against excess light stress: Multiple consequences of light attenuation. Physiol. Plant. 2014;152:585–598. doi: 10.1111/ppl.12201. [DOI] [PubMed] [Google Scholar]
- 63.Bi X., Zhang J., Chen C., Zhang D., Li P., Ma F. Anthocyanin contributes more to hydrogen peroxide scavenging than other phenolics in apple peel. Food Chem. 2014;152:205–209. doi: 10.1016/j.foodchem.2013.11.088. [DOI] [PubMed] [Google Scholar]
- 64.Apel K., Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant. Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 65.Leopoldini M., Russo N., Chiodo S., Toscano M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006;54:6343–6351. doi: 10.1021/jf060986h. [DOI] [PubMed] [Google Scholar]
- 66.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61:1–21. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dai J., Mumper R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurek J. Alkaloids—Their importance in nature and for human life. In: Kurek J., editor. Their Importance in Nature and for Human Life. IntechOpen; London, UK: 2019. pp. 1–7. [DOI] [Google Scholar]
- 69.Levin D.A. Alkaloids bearing plants: An ecogeographic perspective. Am. Nat. 1976;110:261–284. doi: 10.1086/283063. [DOI] [Google Scholar]
- 70.Nowacki E., Waller G.R. A study on the origin and protective function of alkaloids in plants. Flora. 1973;162:108–117. doi: 10.1016/S0367-2530(17)31694-8. [DOI] [Google Scholar]
- 71.Kitamura Y., Yamashita R., Miura H., Watanabe M. Phloem transport of tropane and pyridine alkaloids in Duboisia myoporoides. J. Plant. Physiol. 1993;142:635–637. doi: 10.1016/S0176-1617(11)80411-6. [DOI] [Google Scholar]
- 72.Wangchuk P. Plant alkaloids: Classification, isolation and drug development. In: Swamy M.K., Patra J.K., Rudramurthy G.R., editors. Medicinal Plants: Chemistry, Pharmacology and Therapeutic Applications. Taylor & Francis Ltd.; Boca Raton, FL, USA: 2019. pp. 131–137. [Google Scholar]
- 73.Saunders J.A., O’Neill N.R., Romeo J.T. Alkaloids chemistry and feeding specificity of insect herbivores. In: Pelletier S.W., editor. Alkaloids: Chemical and Biological Perspectives. Springer; New York, NY, USA: 1992. pp. 151–196. [Google Scholar]
- 74.Bastias D.A., Martinez-Ghersa M.A., Ballare C.L., Gundel P.E. Epichloe fungal endophytes and plant defenses: Not just alkaloids. Trends Plant. Sci. 2017;22:939–948. doi: 10.1016/j.tplants.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 75.Bastias D.A., Gianoli E., Gundel P.E. Fungal endophytes can eliminate the plant growth–defence trade- off. New Phytol. 2021;230:2105–2113. doi: 10.1111/nph.17335. [DOI] [PubMed] [Google Scholar]
- 76.McCulley R.L., Bush L.P., Carlisle A.E., Ji H., Nelson J.A. Warming reduces tall fescue abundance but stimulates toxic alkaloid concentrations in transition zone pastures of the U.S. Front. Chem. 2014;2:1–14. doi: 10.3389/fchem.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waller G.R., Nowacki E.K. Alkaloid Biology and Metabolism in Plants. Springer; Boston, MA, USA: 1978. pp. 85–119. [Google Scholar]
- 78.Vetter J. Plant cyanogenic glycosides. Toxicon. 2000;38:11–36. doi: 10.1016/S0041-0101(99)00128-2. [DOI] [PubMed] [Google Scholar]
- 79.Halkier B.A., Gershenzon J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- 80.Gleadow R.M., Moller B.L. Cyanogenic glycosides: Synthesis, physiology, and phenotypic plasticity. Annu. Rev. Plant Biol. 2014;65:155–185. doi: 10.1146/annurev-arplant-050213-040027. [DOI] [PubMed] [Google Scholar]
- 81.Nyirenda K.K. Toxicity potential of cyanogenic glycosides in edible plants. In: Erkekoglu P., Ogawa T., editors. Medical Toxicology. IntechOpen Limited; London, UK: 2020. pp. 1–19. [DOI] [Google Scholar]
- 82.Cho H.J., Do B.K., Shim S.M., Kwon H., Lee D.H., Nah A.H., Choi Y.J., Lee S.Y. Determination of cyanogenic compounds in edible plants by ion chromatography. Toxicol. Res. 2013;29:143–147. doi: 10.5487/TR.2013.29.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gleadow R.M., Woodrow I.E. Temporal and spatial variation in cyanogenic glycosides in Eucalyptus cladocalyx. Tree Physiol. 2000;20:591–598. doi: 10.1093/treephys/20.9.591. [DOI] [PubMed] [Google Scholar]
- 84.Miller R.E., Jensen R., Woodrow I.E. Frequency of cyanogenesis in tropical rainforests of far north Queensland, Australia. Ann. Bot. 2006;97:1017–1044. doi: 10.1093/aob/mcl048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hansen C.C., Sorensen M., Veiga T.A.M., Zibrandtsen J.F.S., Heskes A.M., Olsen C.E., Boughton B.A., Moller B.L., Neilson E.H.J. Reconfigured Cyanogenic Glucoside Biosynthesis in Eucalyptus cladocalyx Involves a Cytochrome P450 CYP706C55. Plant Physiol. 2018;178:1081–1095. doi: 10.1104/pp.18.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yulvianti M., Zidorn C. Chemical Diversity of Plant Cyanogenic Glycosides: An Overview of Reported Natural Products. Molecules. 2021;26:719. doi: 10.3390/molecules26030719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vickers C.E., Gershenzon J., Lerdau M.T., Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009;5:283–291. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- 88.Wright G.A., Schiestl F.P. The evolution of floral scent: The influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct. Ecol. 2009;23:841–851. doi: 10.1111/j.1365-2435.2009.01627.x. [DOI] [Google Scholar]
- 89.Munne-Bosch S., Falk J. New insights into the function of tocopherols in plants. Planta. 2004;218:323–326. doi: 10.1007/s00425-003-1126-0. [DOI] [PubMed] [Google Scholar]
- 90.Munné-Bosch S., Alegre L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2010;21:31–57. doi: 10.1080/0735-260291044179. [DOI] [Google Scholar]
- 91.Yeshi K., Wangchuk P. Essential oils and their bioactive molecules in healthcare. 1st ed. In: Mandal S.C., Nayak A.K., Dhara A.K., editors. Herbal Biomolecules in Healthcare Applications. Academic Press; London, UK: 2021. pp. 215–237. [Google Scholar]
- 92.Peñuelas J., Llusià J. The complexity of factors driving volatile organic compound emissions by plants. Biol. Plant. 2001;44:481–487. doi: 10.1023/A:1013797129428. [DOI] [Google Scholar]
- 93.Dudareva N., Negre F., Nagegowda D.A., Orlova I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006;25:417–440. doi: 10.1080/07352680600899973. [DOI] [Google Scholar]
- 94.Janas K.M., Cvikrová M., Pałagiewicz A., Szafranska K., Posmyk M.M. Constitutive elevated accumulation of phenylpropanoids in soybean roots at low temperature. Plant Sci. 2002;163:369–373. doi: 10.1016/S0168-9452(02)00136-X. [DOI] [Google Scholar]
- 95.Ono M., Takeshima M., Nishi A., Higuchi T., Nakano S. Genistein suppresses v-Src-driven proliferative activity by arresting the cell-cycle at G2/M through increasing p21 level in Src-activated human gallbladder carcinoma cells. Nutr. Cancer. 2020:1–9. doi: 10.1080/01635581.2020.1797835. [DOI] [PubMed] [Google Scholar]
- 96.Choi E.J., Kim G.H. Antiproliferative activity of daidzein and genistein may be related to ERalpha/c-erbB-2 expression in human breast cancer cells. Mol. Med. Rep. 2013;7:781–784. doi: 10.3892/mmr.2013.1283. [DOI] [PubMed] [Google Scholar]
- 97.Copolovici L., Kannaste A., Pazouki L., Niinemets U. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J. Plant. Physiol. 2012;169:664–672. doi: 10.1016/j.jplph.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 98.Dutta A., Sen J., Deswal R. Downregulation of terpenoid indole alkaloid biosynthetic pathway by low temperature and cloning of a AP2 type C-repeat binding factor (CBF) from Catharanthus roseus (L). G. Don. Plant Cell Rep. 2007;26:1869–1878. doi: 10.1007/s00299-007-0383-y. [DOI] [PubMed] [Google Scholar]
- 99.Goboza M., Aboua Y.G., Chegou N., Oguntibeju O.O. Vindoline effectively ameliorated diabetes-induced hepatotoxicity by docking oxidative stress, inflammation and hypertriglyceridemia in type 2 diabetes-induced male Wistar rats. Biomed. Pharmacother. 2019;112:1–11. doi: 10.1016/j.biopha.2019.108638. [DOI] [PubMed] [Google Scholar]
- 100.Kubo A., Lunde C.S., Kubo I. Indole and (E)-2-hexenal, phytochemical potentiators of polymyxins against Pseudomonas aeruginosa and Escherichia coli. Antimicrob. Agents Chemother. 1996;40:1438–1441. doi: 10.1128/AAC.40.6.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang X.-S., Yang W., Tao S.-J., Li K., Li M., Dong J.-H., Wang M.-W. The effect of delta-elemene on hela cell lines by apoptosis induction. Yakugaku Zasshi. 2006;126:979–990. doi: 10.1248/yakushi.126.979. [DOI] [PubMed] [Google Scholar]
- 102.Legault J., Pichette A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007;59:1643–1647. doi: 10.1211/jpp.59.12.0005. [DOI] [PubMed] [Google Scholar]
- 103.Dahham S.S., Tabana Y.M., Khadeer Ahamed M.B., Abdul Majid A.M.S. In vivo anti-inflammatory activity of β-caryophyllene, evaluated by molecular imaging. Molecules Med. Chem. 2016;162:108–117. doi: 10.14800/mmc.1001. [DOI] [Google Scholar]
- 104.Christie P.J., Alfenito M.R., Walbot V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta. 1994;194:541–549. doi: 10.1007/BF00714468. [DOI] [Google Scholar]
- 105.Ku S.K., Yoon E.K., Lee W., Kwon S., Lee T., Bae J.S. Antithrombotic and antiplatelet activities of pelargonidin in vivo and in vitro. Arch. Pharm. Res. 2016;39:398–408. doi: 10.1007/s12272-016-0708-x. [DOI] [PubMed] [Google Scholar]
- 106.Jeon J., Kim J.K., Wu Q., Park S.U. Effects of cold stress on transcripts and metabolites in tartary buckwheat (Fagopyrum tataricum) Environ. Exp. Bot. 2018;155:488–496. doi: 10.1016/j.envexpbot.2018.07.027. [DOI] [Google Scholar]
- 107.Khoo H.E., Azlan A., Nurulhuda M.H., Ismail A., Abas F., Hamid M., Roowi S. Antioxidative and cardioprotective properties of anthocyanins from defatted dabai extracts. Evid. Based Complement. Alternat. Med. 2013;2013:1–13. doi: 10.1155/2013/434057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mir B.A., Mir S.A., Khazir J., Tonfack L.B., Cowan D.A., Vyas D., Koul S. Cold stress affects antioxidative response and accumulation of medicinally important withanolides in Withania somnifera (L.) Dunal. Ind. Crops. Prod. 2015;74:1008–1016. doi: 10.1016/j.indcrop.2015.06.012. [DOI] [Google Scholar]
- 109.Akhoon B.A., Pandey S., Tiwari S., Pandey R. Withanolide A offers neuroprotection, ameliorates stress resistance and prolongs the life expectancy of Caenorhabditis elegans. Exp. Gerontol. 2016;78:47–56. doi: 10.1016/j.exger.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 110.Hahm E.R., Moura M.B., Kelley E.E., Van Houten B., Shiva S., Singh S.V. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS ONE. 2011;6:1–12. doi: 10.1371/journal.pone.0023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao M., Zhang N., Gao T., Jin J., Jing T., Wang J., Wu Y., Wan X., Schwab W., Song C. Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New Phytol. 2020;226:362–372. doi: 10.1111/nph.16364. [DOI] [PubMed] [Google Scholar]
- 112.Sarker U., Oba S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant. Biol. 2018;18:1–15. doi: 10.1186/s12870-018-1484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Akojie F.O., Fung L.W. Antisickling activity of hydroxybenzoic acids in Cajanus cajan. Planta Med. 1992;58:317–320. doi: 10.1055/s-2006-961475. [DOI] [PubMed] [Google Scholar]
- 114.Hernandez I., Alegre L., Munne-Bosch S. Enhanced oxidation of flavan-3-ols and proanthocyanidin accumulation in water-stressed tea plants. Phytochemistry. 2006;67:1120–1126. doi: 10.1016/j.phytochem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 115.Anitha S., Krishnan S., Senthilkumar K., Sasirekha V. Theoretical investigation on the structure and antioxidant activity of (+) catechin and (−) epicatechin—A comparative study. Mol. Phys. 2020;118:1–12. doi: 10.1080/00268976.2020.1745917. [DOI] [Google Scholar]
- 116.Liu Z. Drought-induced in vivo synthesis of camptothecin in Camptotheca acuminata seedlings. Physiol. Plant. 2000;110:483–488. doi: 10.1111/j.1399-3054.2000.1100409.x. [DOI] [Google Scholar]
- 117.Adams D.J., Dewhirst M.W., Flowers J.L., Gamcsik M.P., Colvin O.M., Manikumar G., Wani M.C., Wall M.E. Camptothecin analogues with enhanced antitumor activity at acidic pH. Cancer Chemother. Pharmacol. 2000;46:263–271. doi: 10.1007/s002800000157. [DOI] [PubMed] [Google Scholar]
- 118.Liu Y., Meng Q., Duan X., Zhang Z., Li D. Effects of PEG-induced drought stress on regulation of indole alkaloid biosynthesis in Catharanthus roseus. J. Plant Interact. 2017;12:87–91. doi: 10.1080/17429145.2017.1293852. [DOI] [Google Scholar]
- 119.Zhou X., Xu Z., Li A., Zhang Z., Xu S. Double-sides sticking mechanism of vinblastine interacting with alpha, beta-tubulin to get activity against cancer cells. J. Biomol. Struct. Dyn. 2019;37:4080–4091. doi: 10.1080/07391102.2018.1539412. [DOI] [PubMed] [Google Scholar]
- 120.Hernandez I., Alegre L., Munne-Bosch S. Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under Mediterranean field conditions. Tree Physiol. 2004;24:1303–1311. doi: 10.1093/treephys/24.11.1303. [DOI] [PubMed] [Google Scholar]
- 121.Rao S.D., Pagidas K. Epigallocatechin-3-gallate, a natural polyphenol, inhibits cell proliferation and induces apoptosis in human ovarian cancer Cclls. Anticancer Res. 2010;30:2519–2524. [PubMed] [Google Scholar]
- 122.Falcinelli S.D., Shi M.C., Friedlander A.M., Chua J. Green tea and epigallocatechin-3-gallate are bactericidal against Bacillus anthracis. FEMS Microbiol. Lett. 2017;364:1–8. doi: 10.1093/femsle/fnx127. [DOI] [PubMed] [Google Scholar]
- 123.Kirakosyan A., Kaufman P., Warber S., Zick S., Aaronson K., Bolling S., Chul Chang S. Applied environmental stresses to enhance the levels of polyphenolics in leaves of hawthorn plants. Physiol. Plant. 2004;121:182–186. doi: 10.1111/j.1399-3054.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 124.Sato Y., Itagaki S., Kurokawa T., Ogura J., Kobayashi M., Hirano T., Sugawara M., Iseki K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011;403:136–138. doi: 10.1016/j.ijpharm.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 125.Xu J.Z., Yeung S.Y., Chang Q., Huang Y., Chen Z.Y. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br. J. Nutr. 2004;91:873–881. doi: 10.1079/BJN20041132. [DOI] [PubMed] [Google Scholar]
- 126.Cho Y., Njiti V.N., Lightfoot D.A., Wood A.J. Trigonelline concentration in field-grown soybean in response to irrigation. Biol. Plant. 2003;46:405–410. doi: 10.1023/A:1024390522259. [DOI] [Google Scholar]
- 127.Yoshinari O., Sato H., Igarashi K. Anti-diabetic effects of pumpkin and its components, trigonelline and nicotinic acid, on Goto-Kakizaki rats. Biosci. Biotechnol. Biochem. 2009;73:1033–1041. doi: 10.1271/bbb.80805. [DOI] [PubMed] [Google Scholar]
- 128.Nacif de Abreu I., Mazzafera P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005;43:241–248. doi: 10.1016/j.plaphy.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 129.Bridi H., Dischkaln Stolz E., Maikon Corrêa de Barros F., Elingson da Silva Costa B., Guerini L., Maris Kuze Rates S., Lino von Poser G. Antinociceptive activity of phloroglucinol derivatives isolated from southern Brazilian Hypericum species. Chem. Biodivers. 2018;15:66–87. doi: 10.1002/cbdv.201800266. [DOI] [PubMed] [Google Scholar]
- 130.Lin J.P., Yang J.S., Lin J.J., Lai K.C., Lu H.F., Ma C.Y., Sai-Chuen Wu R., Wu K.C., Chueh F.S., Gibson Wood W., et al. Rutin inhibits human leukemia tumor growth in a murine xenograft model in vivo. Environ. Toxicol. 2012;27:480–484. doi: 10.1002/tox.20662. [DOI] [PubMed] [Google Scholar]
- 131.Zuco V., Supino R., Righetti S.C., Cleris L., Marchesi E., Gambacorti-Passerini C., Formelli F. Selective cytotoxicity of betulinic acid on tumor cell lines but not on normal cells. Cancer Lett. 2002;175:17–25. doi: 10.1016/S0304-3835(01)00718-2. [DOI] [PubMed] [Google Scholar]
- 132.Christiansen J.L., Jornsgard B., Buskov S., Oslen C.E. Effect of drought stress on content and composition of seed alkaloids in narrow-leafed lupin, Lupinus angustifolius L. Eur. J. Agron. 1997;7:307–314. doi: 10.1016/S1161-0301(97)00017-8. [DOI] [Google Scholar]
- 133.Szabó B., Tyihák E., Szabó G., Botz L. Mycotoxin and drought stress induced change of alkaloid content of Papaver somniferum plantlets. Acta. Bot. Hung. 2003;45:409–417. doi: 10.1556/ABot.45.2003.3-4.15. [DOI] [Google Scholar]
- 134.Stein C., Comisel K., Haimerl E., Yassouridis A., Lehrberger K., Herz A., Peter K. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N. Engl. J. Med. 1991;325:1123–1126. doi: 10.1056/NEJM199110173251602. [DOI] [PubMed] [Google Scholar]
- 135.Clark E., Plint A.C., Correll R., Gaboury I., Passi B. A randomized, controlled trial of acetaminophen, ibuprofen, and codeine for acute pain relief in children with musculoskeletal trauma. Pediatrics. 2007;119:460–467. doi: 10.1542/peds.2006-1347. [DOI] [PubMed] [Google Scholar]
- 136.Turtola S., Manninen A.M., Rikala R., Kainulainen P. Drought stress alters the concentration of wood terpenoids in Scots pine and Norway spruce seedlings. J. Chem. Ecol. 2003;29:1981–1995. doi: 10.1023/A:1025674116183. [DOI] [PubMed] [Google Scholar]
- 137.Ulusu N.N., Ercil D., Sakar M.K., Tezcan E.F. Abietic acid inhibits lipoxygenase activity. Phytother Res. 2002;16:88–90. doi: 10.1002/ptr.983. [DOI] [PubMed] [Google Scholar]
- 138.Fernandez M.A., Tornos M.P., GarcIa M.D., de las Heras B., Villar A.M., Saenz M.T. Anti-inflammatory activity of abietic acid, a diterpene isolated from Pimenta racemosa var. grissea. J. Pharm. Pharmacol. 2001;53:867–872. doi: 10.1211/0022357011776027. [DOI] [PubMed] [Google Scholar]
- 139.Liu H., Wang X., Wang D., Zou Z., Liang Z. Effect of drought stress on growth and ccumulation of active constituents in Salvia miltiorrhiza Bunge. Ind. Crops. Prod. 2011;33:84–88. doi: 10.1016/j.indcrop.2010.09.006. [DOI] [Google Scholar]
- 140.Chen W., Lu Y., Chen G., Huang S. Molecular evidence of cryptotanshinone for treatment and prevention of human cancer. Anticancer Agents Med. Chem. 2013;13:979–987. doi: 10.2174/18715206113139990115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Adomako-Bonsu A.G., Chan S.L.F., Pratten M., Fry J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. In Vitro. 2017;40:248–255. doi: 10.1016/j.tiv.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 142.Sun Y., Zhu H., Wang J., Liu Z., Bi J. Isolation and purification of salvianolic acid A and salvianolic acid B from Salvia miltiorrhiza by high-speed counter-current chromatography and comparison of their antioxidant activity. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009;877:733–737. doi: 10.1016/j.jchromb.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 143.Wang D.H., Du F., Liu H.Y., Liang Z.S. Drought stress increases iridoid glycosides biosynthesis in the roots of Scrophularia ningpoensis seedlings. J. Med. Plant. Res. 2010;4:2691–2699. [Google Scholar]
- 144.Liu L., Cao X., Li T., Li X. Effects of catalpol on the activity of human liver cytochrome P450 enzymes. Xenobiotica. 2019;49:1289–1295. doi: 10.1080/00498254.2018.1558309. [DOI] [PubMed] [Google Scholar]
- 145.Song M., Han M., Kim Kwon Y. Effect of aucubin on neural precursor cell survival during neuronal differentiation. Int. J. Neurosci. 2018;128:899–905. doi: 10.1080/00207454.2018.1435535. [DOI] [PubMed] [Google Scholar]
- 146.Marquardt D., Williams J.A., Kucerka N., Atkinson J., Wassall S.R., Katsaras J., Harroun T.A. Tocopherol activity correlates with its location in a membrane: A new perspective on the antioxidant vitamin E. J. Am. Chem Soc. 2013;135:7523–7533. doi: 10.1021/ja312665r. [DOI] [PubMed] [Google Scholar]
- 147.Kawata A., Murakami Y., Suzuki S., Fujisawa S. Anti-inflammatory activity of beta-carotene, lycopene and tri-n-butylborane, a scavenger of reactive oxygen species. In Vivo. 2018;32:255–264. doi: 10.21873/invivo.11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rufino A.T., Ribeiro M., Judas F., Salgueiro L., Lopes M.C., Cavaleiro C., Mendes A.F. Anti-inflammatory and chondroprotective activity of (+)-alpha-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014;77:264–269. doi: 10.1021/np400828x. [DOI] [PubMed] [Google Scholar]
- 149.Du Y., Luan J., Jiang R.P., Liu J., Ma Y. Myrcene exerts anti-asthmatic activity in neonatal rats via modulating the matrix remodeling. Int. J. Immunopathol. Pharmacol. 2020;34:1–10. doi: 10.1177/2058738420954948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roberto D., Micucci P., Sebastian T., Graciela F., Anesini C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic. Clin. Pharmacol. Toxicol. 2010;106:38–44. doi: 10.1111/j.1742-7843.2009.00467.x. [DOI] [PubMed] [Google Scholar]
- 151.Fernandes E.S., Passos G.F., Medeiros R., da Cunha F.M., Ferreira J., Campos M.M., Pianowski L.F., Calixto J.B. Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007;569:228–236. doi: 10.1016/j.ejphar.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 152.He X., Huang W., Chen W., Dong T., Liu C., Chen Z., Xu S., Ruan Y. Changes of main secondary metabolites in leaves of Ginkgo biloba in response to ozone fumigation. J. Environ. Sci. 2009;21:199–203. doi: 10.1016/S1001-0742(08)62251-2. [DOI] [PubMed] [Google Scholar]
- 153.Feng Z., Sun Q., Chen W., Bai Y., Hu D., Xie X. The neuroprotective mechanisms of ginkgolides and bilobalide in cerebral ischemic injury: A literature review. Mol. Med. 2019;25:1–8. doi: 10.1186/s10020-019-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ormrod D.P., Landry L.G., Coclin P.L. Short-term UV-B radiation and ozone exposure effects on aromatic secondary metabolite accumulation and shoot growth of flavonoid-deficient Arabidopsis mutants. Physiol. Plant. 1995;93:602–610. doi: 10.1111/j.1399-3054.1995.tb05106.x. [DOI] [Google Scholar]
- 155.Olsson L.C., Veit M., Weissenbock G., Bornman J.F. Flavonoid response to UV-B radiation in Brassica napus. Phytochemistry. 1998;49:1021–1028. doi: 10.1016/S0031-9422(98)00062-4. [DOI] [Google Scholar]
- 156.Gitz D.C., Liu L., McClure J.W. Phenolic metabolism, growth and UV-B tolerance in phenylalanine ammonia lyase inhibited red cabbage seedlings. Phytochemistry. 1998;49:377–386. doi: 10.1016/S0031-9422(98)00011-9. [DOI] [Google Scholar]
- 157.Ramani S., Jayabaskaran C. Enhanced catharanthine and vindoline production in suspension cultures of Catharanthus roseus by ultraviolet-B light. J. Mol. Signal. 2008;3:1–6. doi: 10.1186/1750-2187-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ramani S., Chelliah J. UV-B-induced signaling events leading to enhanced-production of catharanthine in Catharanthus roseus cell suspension cultures. BMC Plant. Biol. 2007;7:1–17. doi: 10.1186/1471-2229-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Siddiqui M.J., Ismail Z., Aisha A.F.A., Abdul Majid A.M.S. Cytotoxic activity of Catharanthus roseus (Apocynaceae) crude extracts and pure compounds against human colorectal carcinoma cell line. Int. J. Pharmacol. 2010;6:43–47. doi: 10.3923/ijp.2010.43.47. [DOI] [Google Scholar]
- 160.Wang J., Dudareva N., Bhakta S., Raguso R.A., Pichersky E. Floral scent production in Clarkia breweri (Onagraceae). II. Localization and developmental modulation of the enzyme SAM:(Iso)eugenol O-methyltransferase and phenylpropanoid emission. Plant. Physiol. 1997;114:213–221. doi: 10.1104/pp.114.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Campaniello D., Corbo M.R., Sinigaglia M. Antifungal activity of eugenol against Penicillium, Aspergillus, and Fusarium species. J. Food Prot. 2010;73:1124–1128. doi: 10.4315/0362-028X-73.6.1124. [DOI] [PubMed] [Google Scholar]
- 162.Lee Y.Y., Hung S.L., Pai S.F., Lee Y.H., Yang S.F. Eugenol suppressed the expression of lipopolysaccharide-induced proinflammatory mediators in human macrophages. J. Endod. 2007;33:698–702. doi: 10.1016/j.joen.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 163.Regvar M., Bukovnik U., Likar M., Kreft I. UV-B radiation affects flavonoids and fungal colonisation in Fagopyrum esculentum and F. tataricum. Open Life Sci. 2012;7:275–283. doi: 10.2478/s11535-012-0017-4. [DOI] [Google Scholar]
- 164.Zheng Y.Z., Deng G., Liang Q., Chen D.F., Guo R., Lai R.C. Antioxidant activity of quercetin and its glucosides from Propolis: A theoretical study. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-08024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sun H., Yin M., Hao D., Shen Y. Anti-Cancer Activity of Catechin against A549 Lung Carcinoma Cells by Induction of Cyclin Kinase Inhibitor p21 and Suppression of Cyclin E1 and P–AKT. Appl. Sci. 2020;10:2065. doi: 10.3390/app10062065. [DOI] [Google Scholar]
- 166.Simos Y.V., Verginadis I.I., Toliopoulos I.K., Velalopoulou A.P., Karagounis I.V., Karkabounas S.C., Evangelou A.M. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox. Rep. 2012;17:181–186. doi: 10.1179/1351000212Y.0000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cuadra P., Harborne J.B., Waterman P.G. Increases in surface flavonols and photosynthetic pigments in Gnaphalium luteo-album in response to UV-B radiation. Phytochemistry. 1997;45:1377–1383. doi: 10.1016/S0031-9422(97)00183-0. [DOI] [Google Scholar]
- 168.Faham N., Javidnia K., Bahmani M., Amirghofran Z. Calycopterin, an immunoinhibitory compound from the extract of Dracocephalum kotschyi. Phytother. Res. 2008;22:1154–1158. doi: 10.1002/ptr.2382. [DOI] [PubMed] [Google Scholar]
- 169.Cuadra P., Harborne J.B. Changes in epicuticular flavonoids and photosynthetic pigments as a plant response to UV-B radiation. Z. Naturforsch. 1996;51c:671–680. doi: 10.1515/znc-1996-9-1012. [DOI] [Google Scholar]
- 170.Reuber S., Bornman J.F., Weissenbock G. A flavonoid mutant of barley exhibits increased sensitivity to UV-B radiation in primary leaves. Plant Cell Environ. 1996;19:593–599. doi: 10.1111/j.1365-3040.1996.tb00393.x. [DOI] [Google Scholar]
- 171.Ra J.-E., Woo S.-Y., Jin H., Lee M.J., Kim H.Y., Ham H., Chung I.-M., Seo W.D. Evaluation of antihypertensive polyphenols of barley (Hordeum vulgare L.) seedlings via their effects on angiotensin-converting enzyme (ACE) inhibition. Appl. Biol. Chem. 2020;63:1–9. doi: 10.1186/s13765-020-00519-9. [DOI] [Google Scholar]
- 172.Guo Y., Liu Y., Zhang Z., Chen M., Zhang D., Tian C., Liu M., Jiang G. The antibacterial activity and mechanism of action of luteolin against Trueperella pyogenes. Infect. Drug Resist. 2020;13:1697–1711. doi: 10.2147/IDR.S253363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Markham K.R., Ryan K.G., Bloor S.J., Mitchell K.A. An increase in the luteolin-apigenin ratio in Marchantia polymorpha on UV-B enhancement. Phytochemistry. 1998;48:791–794. doi: 10.1016/S0031-9422(97)00875-3. [DOI] [Google Scholar]
- 174.Skaltsa H., Verykokidou E., Harvala C., Karabourniotis G., Manetas Y. UV-B protective potential and flavonoid content of leaf hairs in Quercus ilex. Phytochemistry. 1994;37:987–990. doi: 10.1016/S0031-9422(00)89514-X. [DOI] [Google Scholar]
- 175.Lee K.M., Lee K.W., Jung S.K., Lee E.J., Heo Y.S., Bode A.M., Lubet R.A., Lee H.J., Dong Z. Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity. Biochem. Pharmacol. 2010;80:2042–2049. doi: 10.1016/j.bcp.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kadioglu O., Nass J., Saeed M.E.M., Schuler B., Efferth T. Kaempferol is an anti-inflammatory compound with activity towards NF-κB pathway proteins. Anticancer Res. 2015;35:2645–2650. [PubMed] [Google Scholar]
- 177.Zu Y.G., Tang Z.H., Yu J.H., Liu S.G., Wang W., Guo X.R. Different responses of camptothecin and 10-hydroxycamptothecin to heat shock in Camptotheca acuminata seedlings. Acta. Bot. Sin. 2003;45:809–814. [Google Scholar]
- 178.Ping Y.-H., Lee H.-C., Lee J.-Y., Wu P.-H., Ho L.-K., Chi C.-W., Lu M.-F., Wang J.-J. Anticancer effects of low-dose 10-hydroxycamptothecin in human colon cancer. Oncol. Rep. 2006;15:1273–1279. doi: 10.3892/or.15.5.1273. [DOI] [PubMed] [Google Scholar]
- 179.Rosenfeld H.J., Aaby K., Lea P. Influence of temperature and plant density on sensory quality and volatile terpenoids of carrot (Daucus carota L.) root. J. Sci. Food Agric. 2002;82:1384–1390. doi: 10.1002/jsfa.1200. [DOI] [Google Scholar]
- 180.Helmig D., Ortega J., Duhl T., Tanner D., Guenther A., Harley P., Wiedinmyer C., Milford J., Sakulyanontvittaya T. Sesquiterpene emissions from pine trees-identifications, emission rates and flux estimates for the contiguous United States. Environ. Sci. Technol. 2007;41:1545–1553. doi: 10.1021/es0618907. [DOI] [PubMed] [Google Scholar]
- 181.Commisso M., Toffali K., Strazzer P., Stocchero M., Ceoldo S., Baldan B., Levi M., Guzzo F. Impact of phenylpropanoid compounds on heat stress tolerance in carrot cell cultures. Front. Plant Sci. 2016;7:1–16. doi: 10.3389/fpls.2016.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Aydin E., Turkez H., Tasdemir S. Anticancer and antioxidant properties of terpinolene in rat brain cells. Arh. Hig. Rada. Toksikol. 2013;64:415–424. doi: 10.2478/10004-1254-64-2013-2365. [DOI] [PubMed] [Google Scholar]
- 183.Shen Y., Song X., Li L., Sun J., Jaiswal Y., Huang J., Liu C., Yang W., Williams L., Zhang H., et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019;111:579–587. doi: 10.1016/j.biopha.2018.12.074. [DOI] [PubMed] [Google Scholar]
- 184.Spagnol C.M., Assis R.P., Brunetti I.L., Isaac V.L.B., Salgado H.R.N., Correa M.A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim Acta A Mol. Biomol. Spectrosc. 2019;219:358–366. doi: 10.1016/j.saa.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 185.Hanson D.T., Sharkey T.D. Effect of growth conditions on isoprene emission and other thermotolerance-enhancing compounds. Plant Cell Environ. 2001;24:929–936. doi: 10.1046/j.1365-3040.2001.00744.x. [DOI] [Google Scholar]
- 186.Zhang J.H., Sun H.L., Chen S.Y., Zeng L., Wang T.T. Anti-fungal activity, mechanism studies on alpha-phellandrene and nonanal against Penicillium cyclopium. Bot. Stud. 2017;58:1–9. doi: 10.1186/s40529-017-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hadri A., Gomez del Rio M.A., Sanz J., Coloma A.G., Idaomar M., Ozonas B.R., Gonzalez J.B., Reus M.I.S. Cytotoxic activity of α-humulene and trans-caryophyllene from Salvia officinalis in animal and human tumor cells. An. R. Acad. Nac. Farm. 2010;76:343–356. [Google Scholar]
- 188.Randriamampionona D., Diallo B., Rakotoniriana F., Rabemanantsoa C., Cheuk K., Corbisier A.M., Jaziri M. Comparative analysis of active constituents in Centella asiatica samples from Madagascar: Application for ex situ conservation and clonal propagation. Fitoterapia. 2007;78:482–489. doi: 10.1016/j.fitote.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 189.Sondari D., Harmami S.B., Ghozali M., Randy A., Amanda A., Irawan Y. Determination of the active asiaticoside content in Centella asiatica as anti-cellulite agent. Indones J. Cancer Chemoprev. 2011;2:222–227. doi: 10.14499/indonesianjcanchemoprev2iss2pp222-227. [DOI] [Google Scholar]
- 190.Sewelam N., Kazan K., Schenk P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant. Sci. 2016;7:1–21. doi: 10.3389/fpls.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nogués I., Medori M., Calfapietra C. Limitations of monoterpene emissions and their antioxidant role in Cistus sp. under mild and severe treatments of drought and warming. Environ. Exp. Bot. 2015;119:76–86. doi: 10.1016/j.envexpbot.2015.06.001. [DOI] [Google Scholar]
- 192.Fall R., Karl T., Hansel A., Jordan A., Lindinger W. Volatile organic compounds emitted after leaf wounding: On-line analysis by proton-transfer-reaction mass spectrometry. J. Geophys. Res. 1999;104:15963–15974. doi: 10.1029/1999JD900144. [DOI] [Google Scholar]
- 193.D’Auria J.C., Pichersky E., Schaub A., Hansel A., Gershenzon J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 2007;49:194–207. doi: 10.1111/j.1365-313X.2006.02946.x. [DOI] [PubMed] [Google Scholar]
- 194.Yamauchi Y., Kunishima M., Mizutani M., Sugimoto Y. Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Sci. Rep. 2015;5:1–8. doi: 10.1038/srep08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gibbs A.G. Lipid melting and cuticular permeability: New insights into an old problem. J. Insect Physiol. 2002;48:391–400. doi: 10.1016/S0022-1910(02)00059-8. [DOI] [PubMed] [Google Scholar]
- 196.Boncan D.A.T., Tsang S.S.K., Li C., Lee I.H.T., Lam H.M., Chan T.F., Hui J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020;21:7382. doi: 10.3390/ijms21197382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Korankye E.A., Lada R.R., Asiedu S., Caldwell C. Plant senescence: The role of volatile terpene compounds (VTCs) Am. J. Plant. Sci. 2017;8:3120–3139. doi: 10.4236/ajps.2017.812211. [DOI] [Google Scholar]
- 198.Valifard M., Mohsenzadeh S., Kholdebarin B., Rowshan V., Niazi A., Moghadam A. Effect of salt stress on terpenoid biosynthesis in Salvia mirzayanii: From gene to metabolite. J. Hortic. Sci. Biotechnol. 2018;94:389–399. doi: 10.1080/14620316.2018.1505443. [DOI] [Google Scholar]
- 199.Spicher L., Glauser G., Kessler F. Lipid antioxidant and galactolipid remodeling under temperature stress in tomato plants. Front Plant Sci. 2016;7:1–12. doi: 10.3389/fpls.2016.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sakuragi Y., Maeda H., Dellapenna D., Bryant D.A. alpha-tocopherol plays a role in photosynthesis and macronutrient homeostasis of the cyanobacterium Synechocystis sp. PCC 6803 that is independent of its antioxidant function. Plant Physiol. 2006;141:508–521. doi: 10.1104/pp.105.074765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Havaux M. Plastoquinone in and beyond photosynthesis. Trends Plant Sci. 2020;25:1252–1265. doi: 10.1016/j.tplants.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 202.Sharkey T.D., Chen X.Y., Yeh S. Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol. 2001;125:2001–2006. doi: 10.1104/pp.125.4.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sharkey T.D., Wiberley A.E., Donohue A.R. Isoprene emission from plants: Why and how. Ann. Bot. 2008;101:5–18. doi: 10.1093/aob/mcm240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Loreto F., Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001;127:1781–1787. doi: 10.1104/pp.010497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rinnan R., Rinnan Å., Faubert P., Tiiva P., Holopainen J.K., Michelsen A. Few long-term effects of simulated climate change on volatile organic compound emissions and leaf chemistry of three subarctic dwarf shrubs. Environ. Exp. Bot. 2011;72:377–386. doi: 10.1016/j.envexpbot.2010.11.006. [DOI] [Google Scholar]
- 206.Harvaux M., Kloppstech K. The protective functions of carotenoid and flavonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants. Planta. 2001;213:953–966. doi: 10.1007/s004250100572. [DOI] [PubMed] [Google Scholar]
- 207.Camejo D., Jimenez A., Alarcon J.J., Torres W., Gomez J.M., Sevilla F. Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct. Plant Biol. 2006;33:177–187. doi: 10.1071/FP05067. [DOI] [PubMed] [Google Scholar]
- 208.Al-Huqail A., El-Dakak R.M., Sanad M.N., Badr R.H., Ibrahim M.M., Soliman D., Khan F. Effects of climate temperature and water stress on plant growth and accumulation of antioxidant compounds in Sweet Basil (Ocimum basilicum L.) leafy vegetable. Scientifica. 2020;2020:1–12. doi: 10.1155/2020/3808909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Thakur P., Nayyar H. Facing the Cold Stress by Plants in the Changing Environment: Sensing, Signaling, and Defending Mechanisms. In: Tuteja N., Singh G.S., editors. Plant. Acclimation to Environmental Stress. Springer; New York, NY, USA: 2013. pp. 29–69. [Google Scholar]
- 210.Chinnusamy V., Zhu J., Zhu J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007;12:444–451. doi: 10.1016/j.tplants.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 211.Krol A., Amarowicz R., Weidner S. The effects of cold stress on the phenolic compounds and antioxidant capacity of grapevine (Vitis vinifera L.) leaves. J. Plant Physiol. 2015;189:97–104. doi: 10.1016/j.jplph.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 212.Chaves M.M., Flexas J., Pinheiro C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ahmed S., Griffin T.S., Kraner D., Schaffner M.K., Sharma D., Hazel M., Leitch A.R., Orians C.M., Han W., Stepp J.R., et al. Environmental Factors Variably Impact Tea Secondary Metabolites in the Context of Climate Change. Front. Plant. Sci. 2019;10:1–16. doi: 10.3389/fpls.2019.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dat J., Vandenabeele S., Vranova E., Van Montagu M., Inze D., Van Breusegem F. Dual action of the active oxygen species during plant stress responses. Cell Mol. Life Sci. 2000;57:779–795. doi: 10.1007/s000180050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Overmyer K., Brosche M., Kangasjarvi J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003;8:335–342. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- 216.Nakabayashi R., Mori T., Saito K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal. Behav. 2014;9:1–3. doi: 10.4161/psb.29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rice-Evans C.A., Miller N.J., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant. Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- 218.Massacci A., Nabiev S.M., Pietrosanti L., Nematov S.K., Chernikova T.N., Thor K., Leipner J. Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2008;46:189–195. doi: 10.1016/j.plaphy.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 219.Nakabayashi R., Yonekura-Sakakibara K., Urano K., Suzuki M., Yamada Y., Nishizawa T., Matsuda F., Kojima M., Sakakibara H., Shinozaki K., et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014;77:367–379. doi: 10.1111/tpj.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ma D., Sun D., Wang C., Li Y., Guo T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014;80:60–66. doi: 10.1016/j.plaphy.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 221.Von Wettberg E.J., Stanton M.L., Whittall J.B. How anthocyanin mutants respond to stress: The need to distinguish between stress tolerance and maximal vigour. Evol. Ecol. Res. 2010;12:457–476. [Google Scholar]
- 222.Li X., Lv X., Wang X., Wang L., Zhang M., Ren M. Effects of abiotic stress on anthocyanin accumulation and grain weight in purple wheat. Crop. Pasture. Sci. 2018;69:1208–1214. doi: 10.1071/CP18341. [DOI] [Google Scholar]
- 223.Sun J., Qiu C., Ding Y., Wang Y., Sun L., Fan K., Gai Z., Dong G., Wang J., Li X., et al. Fulvic acid ameliorates drought stress-induced damage in tea plants by regulating the ascorbate metabolism and flavonoids biosynthesis. BMC Genom. 2020;21:1–13. doi: 10.1186/s12864-020-06815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Muller-Xing R., Xing Q., Goodrich J. Footprints of the sun: Memory of UV and light stress in plants. Front Plant Sci. 2014;5:1–12. doi: 10.3389/fpls.2014.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Del Valle J.C., Buide M.L., Whittall J.B., Valladares F., Narbona E. UV radiation increases phenolic compound protection but decreases reproduction in Silene littorea. PLoS ONE. 2020;15:1–18. doi: 10.1371/journal.pone.0231611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stapleton A.E., Walbot V. Flavonoids can protect maize DNA from the induction of ultraviolet radiation damage. Plant Physiol. 1994;105:881–889. doi: 10.1104/pp.105.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jordan B.R. Molecular response of plant cells to UV-B stress. Funct. Plant Biol. 2002;29:909–916. doi: 10.1071/FP02062. [DOI] [PubMed] [Google Scholar]
- 228.Agati G., Brunetti C., Di Ferdinando M., Ferrini F., Pollastri S., Tattini M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013;72:35–45. doi: 10.1016/j.plaphy.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 229.Tattini M., Gravano E., Pinelli P., Mullinacci N., Romani A. Flavonoids accumulate in leaves and glandular trichomes of phillyrea latifolia exposed to excess solar radiation. New Phytol. 2000;148:69–77. doi: 10.1046/j.1469-8137.2000.00743.x. [DOI] [PubMed] [Google Scholar]
- 230.Tattini M., Galardi C., Pinelli P., Massai R., Remorini D., Agati G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004;163:547–561. doi: 10.1111/j.1469-8137.2004.01126.x. [DOI] [PubMed] [Google Scholar]
- 231.Nascimento L., Leal-Costa M.V., Menezes E.A., Lopes V.R., Muzitano M.F., Costa S.S., Tavares E.S. Ultraviolet-B radiation effects on phenolic profile and flavonoid content of Kalanchoe pinnata. J. Photochem. Photobiol. B. 2015;148:73–81. doi: 10.1016/j.jphotobiol.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 232.Herrlich P., Blattner C., Knebel A., Bender K., Rahmsdorf H.F. Nuclear and non-nuclear targets of genotoxic agents in the induction of gene expression. Shared principles in yeast, rodents, man and plants. Biol. Chem. 1997;378:1217–1229. doi: 10.1515/bchm.1997.378.11.1217. [DOI] [PubMed] [Google Scholar]
- 233.Lindroth R.L. Impacts of elevated atmospheric CO2 and O3 on forests: Phytochemistry, trophic interactions, and ecosystem dynamics. J. Chem. Ecol. 2010;36:2–21. doi: 10.1007/s10886-009-9731-4. [DOI] [PubMed] [Google Scholar]
- 234.Iriti M., Faoro F. Chemical diversity and defence metabolism: How plants cope with pathogens and ozone pollution. Int. J. Mol. Sci. 2009;10:3371–3399. doi: 10.3390/ijms10083371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hoshika Y., Omasa K., Paoletti E. Both ozone exposure and soil water stress are able to induce stomatal sluggishness. Environ. Exp. Bot. 2013;88:19–23. doi: 10.1016/j.envexpbot.2011.12.004. [DOI] [Google Scholar]
- 236.Mao B., Yin H., Wang Y., Zhao T.H., Tian R.R., Wang W., Ye J.S. Combined effects of O3 and UV radiation on secondary metabolites and endogenous hormones of soybean leaves. PLoS ONE. 2017;12:1–16. doi: 10.1371/journal.pone.0183147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kangasj¨arvi J., Talvinen J., Utriaine M., Karjalainen R. Plant defence systems induced by ozone. Plant. Cell Environ. 1994;17:783–794. doi: 10.1111/j.1365-3040.1994.tb00173.x. [DOI] [Google Scholar]
- 238.Sharma Y.K., Davis K.R. Ozone-induced expression of stress-related genes in Arabidopsis thaliana. Plant Physiol. 1994;105:1089–1096. doi: 10.1104/pp.105.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Eckey-Kaltenbach H.E., Ernst D., Heller W., Sandermann H. Biochemical plant response to ozone IV. Cross-induction of defensive pathways in parsley (Petroselinum crispum L.) plants. Plant Physiol. 1994;104:67–74. doi: 10.1104/pp.104.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Martin-Tanguy J. Metabolism and function of polyamines in plants: Recent development (new approaches) Plant Growth Regul. 2001;34:135–148. doi: 10.1023/A:1013343106574. [DOI] [Google Scholar]
- 241.Marchica A., Cotrozzi L., Detti R., Lorenzini G., Pellegrini E., Petersen M., Nali C. The biosynthesis of phenolic compounds is an integrated defence mechanism to prevent ozone injury in Salvia officinalis. Antioxidants. 2020;9:1274. doi: 10.3390/antiox9121274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Peñuelas J., Staudt M. BVOCs and global change. Trends Plant Sci. 2010;15:133–144. doi: 10.1016/j.tplants.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 243.Valkama E., Koricheva J., Oksanen E. Effects of elevated O3, alone and in combination with elevated CO2, on tree leaf chemistry and insect herbivore performance: A meta-analysis. Glob. Chang. Biol. 2007;13:184–201. doi: 10.1111/j.1365-2486.2006.01284.x. [DOI] [Google Scholar]
- 244.Rao M.V., Davis K.R. The physiology of ozone-induced cell death. Planta. 2001;213:682–690. doi: 10.1007/s004250100618. [DOI] [PubMed] [Google Scholar]
- 245.Heiden A.C., Hoffmann T., Kahl J., Kley D., Klockow D., Langebartels C., Mehlhorn H., Sandermann H., Schraudner M., Schuh G., et al. Emission of volatile organic compounds from ozone-exposed plants. Ecolog. Appl. 1999;9:1160–1167. doi: 10.1890/1051-0761(1999)009[1160:EOVOCF]2.0.CO;2. [DOI] [Google Scholar]
- 246.Langebartels C., Kerner K., Leonardi S., Schraudner M., Trost M., Heller W., Sandermann H. Biochemical plant resonses to ozone I: Differential induction of polyamine and ethylene biosynthesis in tobacco. Plant Physiol. 1991;9:882–889. doi: 10.1104/pp.95.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Piao M.J., Kim K.C., Chae S., Keum Y.S., Kim H.S., Hyun J.W. Protective effect of fisetin (3,7,3’,4’-tetrahydroxyflavone) against gamma-irradiation-induced oxidative stress and cell damage. Biomol. Ther. 2013;21:210–215. doi: 10.4062/biomolther.2013.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Maher P. A comparison of the neurotrophic activities of the flavonoid fisetin and some of its derivatives. Free Radic. Res. 2006;40:1105–1111. doi: 10.1080/10715760600672509. [DOI] [PubMed] [Google Scholar]
- 249.Pinto D.M., Blande J.D., Nykanen R., WenXia D., Ner A.M., Holopainen J.K. Ozone degrades common herbivore-induced plant volatiles: Does this affect herbivore prey location by predators and parasitoids? J. Chem. Ecol. 2007;33:683–694. doi: 10.1007/s10886-007-9255-8. [DOI] [PubMed] [Google Scholar]
- 250.Liu J.-H., Kitashiba H., Wang J., Ban Y., Moriguchi T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechn. 2007;24:117–126. doi: 10.5511/plantbiotechnology.24.117. [DOI] [Google Scholar]
- 251.Cragg G.M., Newman D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta Gen. Subj. BBA-GEN SUBJECTS. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Williams C.A., Grayer R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004;12:539–573. doi: 10.1039/b311404j. [DOI] [PubMed] [Google Scholar]
- 253.Melidou M., Riganakos K., Galaris D. Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: The role of iron chelation. Free Radic. Biol. Med. 2005;39:1591–1600. doi: 10.1016/j.freeradbiomed.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 254.Selway J.T. Antiviral activity of flavones and flavans. Prog Clin. Biol. Res. 1986;213:521–536. [PubMed] [Google Scholar]
- 255.Maher P. The flavonoid fisetin promotes nerve cell survival from trophic factor withdrawal by enhancement of proteasome activity. Arch. Biochem. Biophy. 2008;476:139–144. doi: 10.1016/j.abb.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 256.Gupta S.C., Kim J.H., Prasad S., Aggarwal B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sung B., Pandey M.K., Aggarwal B.B. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-κB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IκBα kinase activation. Mol. Pharmacol. 2007;71:1703–1714. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 258.Hussain T., Al-Attas O.S., Alamery S., Ahmed M., Odeibat H.A.M., Alrokayan S. The plant flavonoid, fisetin alleviates cigarette smoke-induced oxidative stress, and inflammation in Wistar rat lungs. J. Food Biochem. 2019;43:1–11. doi: 10.1111/jfbc.12962. [DOI] [PubMed] [Google Scholar]
- 259.Choi I.S., Choi E.Y., Jin J.Y., Park H.R., Choi J.I., Kim S.J. Kaempferol inhibits P. intermedia lipopolysaccharide-induced production of nitric oxide through translational regulation in murine macrophages: Critical role of heme oxygenase-1-mediated ROS reduction. J. Periodontol. 2013;84:545–555. doi: 10.1902/jop.2012.120180. [DOI] [PubMed] [Google Scholar]
- 260.Chen A.Y., Chen Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Calderon-Montano J.M., Burgos-Moron E., Perez-Guerrero C., Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini. Rev. Med. Chem. 2011;11:298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- 262.Kalantari H., Das D.K. Physiological effects of resveratrol. Biofactors. 2010;36:401–406. doi: 10.1002/biof.100. [DOI] [PubMed] [Google Scholar]
- 263.Jiang Z., Gao W., Huang L. Tanshinones, critical pharmacological components in Salvia miltiorhiza. Front. Pharmacol. 2019;10:1–14. doi: 10.3389/fphar.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Oliviera A.B., Dolabela M.F., Braga F.C., Jacome R.L.R.P., Varotti F.P., Povoa M.M. Plant-derived antimalarial agents: New leads and efficient phytomedicines. Part I. Alkaloids. An. Acad. Bras. Cienc. 2009;81:715–740. doi: 10.1590/S0001-37652009000400011. [DOI] [PubMed] [Google Scholar]
- 265.Binder B.Y.K., Peebles C.A.M., Shanks J.V., San K.-Y. The effects of UV-B stress on the production of terpenoid indole alkaloids in Catharanthus roseus hairy roots. Biotechnol. Prog. 2009;25:861–865. doi: 10.1002/btpr.97. [DOI] [PubMed] [Google Scholar]
- 266.Wilson L., Creswell K.M., Chin D. The mechanism of action of vinblastine. Binding of [acetyl-3H]vinblastine to embryonic chick brain tubulin and tubulin from sea urchin sperm tail outer doublet microtubules. Biochemistry. 1975;14:5586–5592. doi: 10.1021/bi00697a008. [DOI] [PubMed] [Google Scholar]
- 267.Zi J., Mafu S., Peters R.J. To gibberellins and beyond! surveying the evolution of (di) terpenoid metabolism. Annu. Rev. Plant Biol. 2014;65:259–286. doi: 10.1146/annurev-arplant-050213-035705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Salminen A., Lehtonen M., Suuronen T., Kaarniranta K., Huuskonen J. Terpenoids: Natural inhibitors of NF-kB signaling with anti-inflammatory and anticancer potential Cell Mol. Life Sci. 2008;65:2979–2999. doi: 10.1007/s00018-008-8103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Geu-Flores F., Sherden N.H., Courdavault V., Burlat V., Glenn W.S., Wu C., Nims E., Cui Y., O’Connor S.E. An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature. 2012;492:138–142. doi: 10.1038/nature11692. [DOI] [PubMed] [Google Scholar]
- 270.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 272.Butler M.S., Robertson A.A., Cooper M.A. Natural product and natural product derived drugs in clinical trials. Nat. Prod. Rep. 2014;31:1612–1661. doi: 10.1039/C4NP00064A. [DOI] [PubMed] [Google Scholar]
- 273.Atanasov A.G., Zotchev S.B., Dirsch V.M., International Natural Product Sciences T., Supuran C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zemp M., Hoelzle M., Haeberli W. Six decades of glacier mass balance observations—A review of the worldwide monitoring network. Ann. Glaciol. 2009;50:101–111. doi: 10.3189/172756409787769591. [DOI] [Google Scholar]
- 275.Cazzolla Gatti R., Callaghan T., Velichevskaya A., Dudko A., Fabbio L., Battipaglia G., Liang J. Accelerating upward treeline shift in the Altai Mountains under last-century climate change. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-44188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Williams S.E., Bolitho E.E., Fox S. Climate change in Australian tropical rainforests: An impending environmental catastrophe. Proc. Biol. Sci. 2003;270:1887–1892. doi: 10.1098/rspb.2003.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Steinbauer M.J., Grytnes J.A., Jurasinski G., Kulonen A., Lenoir J., Pauli H., Rixen C., Winkler M., Bardy-Durchhalter M., Barni E., et al. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature. 2018;556:231–234. doi: 10.1038/s41586-018-0005-6. [DOI] [PubMed] [Google Scholar]
- 278.Toscano S., Trivellini A., Cocetta G., Bulgari R., Francini A., Romano D., Ferrante A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant. Sci. 2019;10:1–17. doi: 10.3389/fpls.2019.01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Vogt T. Phenylpropanoid biosynthesis. Mol. Plant. 2010;3:2–20. doi: 10.1093/mp/ssp106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.