Abstract
Background
During COVID-19 pandemic, several vaccines have been developed such as mRNA vaccines. However, acute pericarditis and myocarditis/myopericarditis cases have been described after mRNA vaccination. The mechanism for the development of cardiac involvement is unknown. Potential mechanism for oxidative stress associated with vaccine-induced heart involvement is unidentified. This study aimed to examine the role of oxidative stress and the heart involvement in young adults vaccinated with COVID-19 mRNA vaccines.
Methods
In this cross-sectional study, a total of 23 participants were included and 10 of these participants were asymptomatic patients (control group). Comparison of the cardiac involvement and control group was made by using troponin I, C-reactive protein (hsCRP), D-dimer levels, and oxidative stress tests including nitric oxide, and imaging techniques (ECG, echocardiography, cardiovascular magnetic resonance).
Results
The median age of acute pericarditis group (10 patients) was 22 years (Q1-Q3: 18.5–31), and the mean age was 24.4±7.5 years. The median age of myopericarditis group (3 patients) was 22 years (Q1–Q3 18.0–25.0), and the mean age was 21.6 ±3.5 years. All the myopericarditis cases were male. The patients with myopericarditis had higher troponin I level, hsCRP, and D-dimer levels (troponin I level; 1600.00 ng/mL; D-dimer; 1.20 μg/mL, hsCRP; 3.0 mg/L, respectively; p < 0.05). Serum nitric oxide levels and OSI (total oxidant status, H2O2/total antioxidant status) were lower in myopericarditis group than the control and acute pericarditis group (p < 0.05). This shows inflammatory and procoagulant state.
Conclusion
Vaccine-induced myopericarditis cases are associated with oxidative stress test abnormality (abnormal NO, OSI levels). However, there is no relationship between NO levels and other oxidative stress tests difference in vaccine-induced acute pericarditis. It is thought that vaccine-induced pericarditis and myopericarditis could have different pathogenesis. This could make it necessary to reassess the second dose of vaccination for vaccine-induced cardiac involvement cases.
Keywords: COVID-19 vaccine, cardiac involvement, oxidative stress
Introduction
Vaccination is a well-known effective method to fight against viral infection. However, vaccines had rare cardiac involvement. According to Vaccine Adverse Event Reporting System (VAERS), vaccine-induced heart adverse events were reported as 0.1% like smallpox, influenza, hepatitis B, or other vaccinations.1
To get the coronavirus disease of 2019 (COVID −19) under control, several different types of vaccines, such as mRNA vaccines, have been developed during the pandemic. As an adverse event, case reports have been published about heart involvement after COVID-19 mRNA vaccines.2–4 The cardiac involvement in those patients contained acute pericarditis and myocarditis/myopericarditis, observed higher among males and young people.5 Because of the serious adverse effect, the vaccine-induced cardiac complications could be worrying. The mechanism for the development of cardiac involvement is unclear. However, there are some arguments about it. Firstly, after mRNA vaccination, the immune system may perceive the mRNA as an antigen and start activation of proinflammatory and immunologic reaction.6,7 The innate immunogenicity and genetic predisposition in certain individuals may be responsible for this.8 Moreover, oxidative stress has been known to contribute to cardiovascular problems in the patients with COVID-19.9–11 Vascular nitric oxide (NO) abnormalities cause endothelial dysfunction occurring with various cardiovascular pathologies. The relationship between oxidative stress and COVID-19 vaccine-induced heart involvement (eg acute pericarditis-myopericarditis) is unknown. Thus, this study aims to examine the role of oxidative stress and the discussed aspects of heart involvement in young adults vaccinated with COVID-19 mRNA vaccines.
Methods
This study was a cross-sectional study. The population of the study consisted of 23 patients vaccinated with COVID-19 mRNA vaccines between August 2021 and October 2021. The age range of the groups was from 18 to 38 years. The patients had no prior heart disease. 13 patients had vaccine-induced heart involvement, whereas other patients (10 patients) had no heart involvement (control group). The study protocol was accepted by Medicana International Ankara Hospital Human Research Ethic Committee (2021/24). Written informed consent was obtained from each patient. The principles of Declaration of Helsinki were followed in this study. The study data is available upon request.
Definition of mRNA Vaccine-Induced Heart Involvement
Vaccine-induced acute pericarditis was accepted as pleuritic chest pain, and availability of pericardial effusion, or blood increased white cell count or increased C-reactive protein level (crp), and normal serum troponin levels. Those cases showed no ECG changes like classical acute pericarditis ECG stages. Typically, this condition appeared following the first or second dose of mRNA vaccine within 3 days.
Vaccine-induced acute myocarditis/myopericarditis was accepted as chest pain, and availability of pericardial effusion, focal perimyocardial echo bright appearance, or/and myocardial segment motion abnormality, increased white cell count/increased C-reactive protein level (crp), elevated d-dimer levels, elevated serum troponin levels. ECG findings of the patients had similar appearance to the findings of those with Stage 1 acute pericarditis. This condition occurred following the first or second dose of the mRNA vaccine within 3 days.
Definition of the Control Group
Following the first or second dose of mRNA vaccine within 3 days, the patients had no complaints (eg, chest pain).
The two groups were compared in terms of biochemical parameters (Troponin I, C-reactive protein (hsCRP), D-dimer levels, and oxidative stress tests including nitric oxide), and imaging techniques (ECG, echocardiography, cardiovascular magnetic resonance).
Oxidative Stress Assays and Oxidative Stress Markers
Total antioxidant status (TAS, mmol Trolox Eq/L), total oxidant status (TOS, mmol H2O2 Eq/L), oxidative stress index (OSI-TOS/TAS), oxonase (PON, U/L), nitric oxide (NO, μmol/L) levels were measured for the patients.12,13
The analyses of serum biochemical oxidative stress marker parameters were performed on an auto analyzer (Mindray BS 300) using commercial kits (RelAssay Diagnostic, Turkey). Venous blood samples were collected from all of the patients. TAS test kit (RelAssay Diagnostic®, Turkey) was used according to the manufacturer’s instructions to measure the TAS levels. 30 μL of the sample was mixed with 500 μL of measurement buffer (reagent 1) in an Eppendorf tube. 2 μL of that mixture was used to measure the absorbance at 660 nm (A1) (NanoDrop® ONE, Thermo Scientific). Then, 75 μL of colored 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) (reagent 2) was added to the mixture, and an incubation performed at 37° C for 5 min (ST 30, NUVE).
TOS test kit (RelAssay Diagnostic®, Turkey) was used according to the manufacturer’s instructions to measure the TOS levels. 75 μL of the sample was mixed with 500 μL of measurement buffer (reagent 1) in an Eppendorf tube. 2 μL of that mixture was used to measure the absorbance at 530 nm (A1) (NanoDrop® ONE, Thermo Scientific). Then, 25 μL of Pro-chromogenic solution (reagent 2) was added to the mixture, and an incubation performed at 37° C for 5 min (ST 30, NUVE).
Echocardiographic Evaluation
Standard TTE was performed in the left lateral decubitus position using Philips Affiniti 50G device (Philips, USA) with a 2.5 MHz transducer to examine two-dimensional images (2-D) and to evaluate M-mode and Doppler values. During operation, depth was 20 cm and dynamic range was 6. M-mode and 2-D images of the left ventricle and Doppler parameters were recorded on the basis of the American Society of Echocardiography and the European Association of Cardiovascular Imaging.14
Echocardiographic heart involvement was accepted as pericardial effusion, focal perimyocardial echo bright appearance, or/and myocardial wall segment abnormality.15
Cardiac MR Scan (CMR)
To evaluate for myocarditis and acute pericarditis, cardiac magnetic resonance imaging was performed using clinical 3-T scanners (SIGNA™ Voyager - 70cm - GE Healthcare, USA). Conventional sequences were used for the acquisition of cardiac function, volumes, mass, and scar imaging. Myocardial T1 and T2 mappings were acquired in a single midventricular short-axis slice, while for T2 mapping, a validated sequence for measurement of myocardial edema was used. Late gadolinium enhancement (LGE) imaging was completed approximately 10 minutes after the administration of 0.1 mmol/kg of body weight of gadobutrol (Gadovist; Bayer).
Statistical Analysis
SPSS for Windows 23 (IBM Inc., Chicago, IL, USA) was used for statistical analyses. In the analysis tables, numeric variables were presented as mean, standard deviation, median and quarter value, and non-numeric deviations were presented as frequencies and percentages.
Shapiro–Wilk test was used to test the normality assumption and because the normality assumption was violated, and Kruskal–Wallis H-test was used to compare groups. Following this test, pairwise comparisons were made using Dunn test.
Fisher-Freeman-Halton exact test was used for categorical variables. The value of p<0.05 was considered significant.
Results
Table 1 presents the comparison of the groups according to age and gender. There was no significant statistical difference between the two groups regarding age, gender (p>0.05). Cardiac involvement cases included younger and male adults mostly. The median age of acute pericarditis was 22 years (Q1-Q3: 18.5–31), and the mean age was 24.4±7.5 years. The median age of myopericarditis was 22 years (Q1-Q3 18.0–25.0) and the mean age was 21.6 ±3.5 years. All myopericarditis cases were male.
Table 1.
The Comparison of Groups According to Age and Gender
| Statistic | Total | Control | Pericarditis | Myopericarditis | P | |
|---|---|---|---|---|---|---|
| Gender | n (%) | |||||
| Female | 7 (30.4) | 4 (57.1) | 3 (42.9) | 0 (0.0) | 0.690f | |
| Male | 16 (69.6) | 6 (40.0) | 7 (40.0) | 3 (20.0) | ||
| Age | M±SD | 23.4±5.1 | 23.0±2.4 | 24.4±7.5 | 21.6±3.5 | |
| Median (Q1-Q3) | 22.5 (19.0–25.0) | 23.0 (21.5–25.0) | 22.0 (18.5–31.0) | 22.0 (18.0–25.0) | 0.790h |
Note: f Fisher-Freeman-Halton Exact Test; h Kruskal–Wallis H-test (exact).
Table 2 shows clinical findings classified according to groups. Troponin I level, crp, d-dimer levels in the acute pericarditis and control groups were normal. However, the patients with myopericarditis had higher troponin I level, crp, and d-dimer levels than the control and acute pericarditis group (p <0.05).
Table 2.
Clinical Findings According to Groups
| Measurement Median (Q1-Q3) | Total | Control | Pericarditis | Myopericarditis | Ph |
|---|---|---|---|---|---|
| Troponin I | 2.00 (1.00–2.00) | 1.50a (1.00–2.00) | 1.00a (1.00–2.00) | 1600.00b (1300.00–1600.00) | 0.007 |
| crp | 0.40 (0.30–0.43) | 0.40a (0.30–0.40) | 0.40a (0.30–0.40) | 3.00b (2.80–3.00) | 0.006 |
| D-dimer | 0.30 (0.30–0.40) | 0.30a (0.30–0.40) | 0.30a (0.20–0.30) | 1.20b (1.00–1.20) | 0.001 |
| NO | 17.10 (13.74–18.38) | 17.40a (15.58–18.27) | 17.21a (15.63–20.00) | 11.25b (11.20–11.25) | 0.014 |
| TOS | 2.55 (1.24–4.08) | 2.55 (1.27–2.97) | 4.00 (1.74–4.88) | 1.22 (1.00–1.22) | 0.051 |
| TAS | 1.34 (1.29–1.42) | 1.40 (1.22–1.75) | 1.31 (1.19–1.36) | 1.40 (1.00–1.40) | 0.516 |
| OSI | 0.24 (0.14–0.31) | 0.26a (0.22–0.34) | 0.25a (0.13–0.36) | 0.05b (0.00–0.05) | 0.033 |
| PON | 396.00 (336.75–483.25) | 415.00 (262.50–551.00) | 380.00 (354.00–466.00) | 404.00 (402.00–404.00) | 0.882 |
| Leukocyte | 7.20 (6.30–9.50) | 6.50 (6.20–7.60) | 11.00 (8.28–11.00) | 7.17 (7.17–7.17) | 0.055 |
Notes: a, bWithin same row, different letters indicate groups statistically different from each other based on post hoc comparisons by Dunn test. Bold Values: There were statistically significant differences in troponin I, crp, D-dimer, NO, and OSI levels in myopericarditis cases (p <0.05). hKruskal–Wallis H-test p-value (exact).
Abbreviations: TAS, total antioxidant status (mmol Trolox Eq/L); TOS, total oxidant status (mmol H2O2 Eq/L); OSI, oxidative stress index (TOS/TAS); PON, Paraoxonase (U/L); NO, nitric oxide (μmol/L).
Moreover, in the oxidative stress tests, serum NO levels and OSI (total oxidant status, H2O2/Total antioxidant status) were lower in myopericarditis patients than the control and acute pericarditis group (p< 0.05).
There was no significant statistical difference among the groups regarding TAS, TOS, PON, and leucocyte measurements (p>0.05).
We observed that vaccine-induced acute pericarditis cases had chest pain, normal serum troponin levels, nonspecific ECG findings, and normal cardiac involvement (Figure 1). However, vaccine induced-acute myopericarditis cases (3 patients) had chest pain, elevated serum troponin levels, ST elevation in ECG, and abnormal cardiac involvement (Figure 1).
Figure 1.
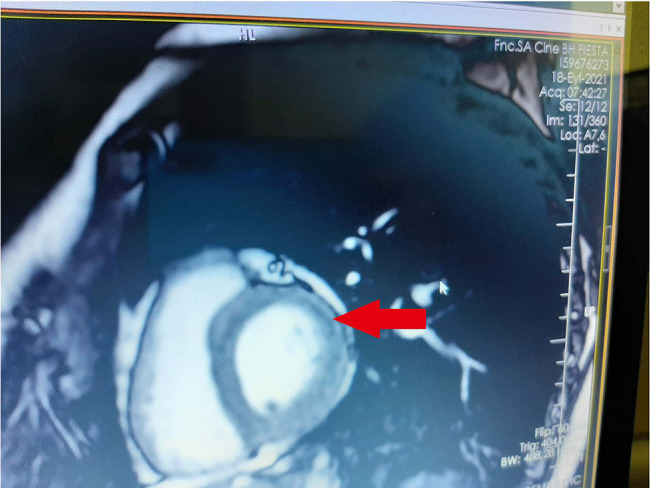
Subepicardial late gadolinium enhancement on CMR.
The patients with myopericarditis were admitted to the hospital. Interestingly, their complaints were rapidly resolved. The ECG results indicated swift recovery (Figure 2A–D).
Figure 2.
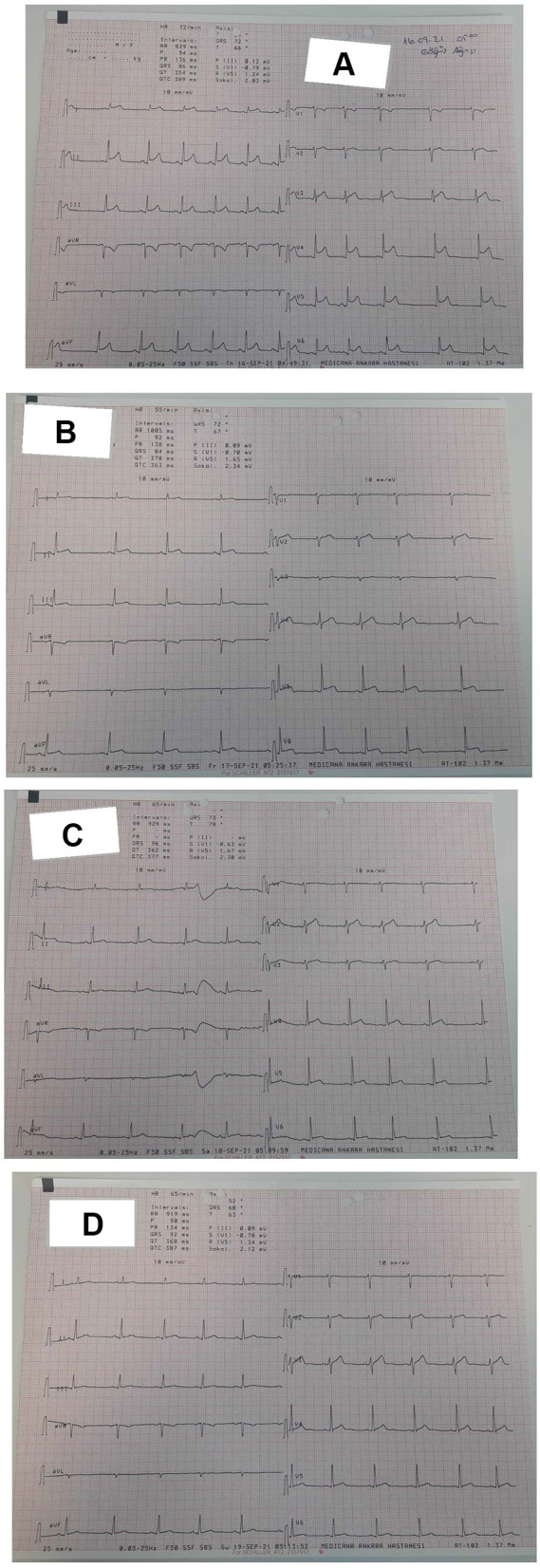
Rapid resolution of ECG findings in patient with acute myopericarditis. (A) At admission, diffuse ST -segment elevation in ECG. (B) Second day at hospital, regression of ST- segment elevation in ECG. (C) Third day at hospital, nearly ST-segment recovery in ECG. (D) Fourth day at hospital, complete ST-segment recovery in ECG.
Discussion
Nanotechnology has an important role in the development of mRNA-based COVID-19 vaccines, including Moderna and Pfizer/BioNTech. The mRNA-based COVID-19 vaccines is based on the scalability of nucleic acid technology.16 These vaccines are both non-infectious and do not require penetration into the nucleus.17 Thanks to nanotechnology, these vaccines can be produced rapidly, which is a major advantage in the existing pandemic.18 However, it is accepted that acute pericarditis and myopericarditis are rare vaccine-induced complications.1 Such adverse effects can be observed in COVID-19 mRNA vaccination as well. The cardiac involvement cases have been detected among individuals roughly between 12 and 39 years of age.5 Seventy-nine percent were in males, with the majority in individuals <30 years of age with a median age of 24.19 According to VAERS, 67% of cases were after the second dose.
Possible mechanism for mRNA vaccine-induced myocarditis is autoantibody generation.20 Another mechanism can be the trigger effect of the preexisting dysregulated pathways in certain individuals with predisposition, resulting in a polyclonal B-cell expansion, immune complex formation, and inflammation.32
COVID-19 associated oxidative stress has been shown in cardiovascular involvement.9 The low levels of NO have been identified at the onset of the post COVID-19 cases. Additionally, the level difference of H2O2 in the COVID-19 patients has been shown to be associated with the lung damage and oxidation of key constituents of innate immunity.21
Other potential causes of myocarditis and pericarditis should be ruled out. There is a need to consider infectious disease and rheumatology-related disease in this evaluation.
In this study, we realized that the patients with myopericarditis had lower NO levels, OSI level compared with acute pericarditis and control groups. Moreover, serum D-dimer levels were fourfold elevated in myopericarditis patients without evidence of pulmonary embolism or venous thromboembolic events. Low NO levels indicate inflammatory and procoagulant state in mRNA vaccine-induced myopericarditis. Therefore, we think that oxidative stress could be the role of mRNA vaccine-induced myopericarditis. However, we found no difference in NO levels and other oxidative stress tests in acute pericarditis cases. This indicates that vaccine-induced pericarditis and myopericarditis could be a different entity.
In this study, the age range of myopericarditis patients were between 18 and 25. Young adult predominance has been defined in other studies.5 However, the reasons are unknown. The age-related immunity changes in human health could explain some reasons, which the decline in CD4 T cell and dendritic cell are functioning, the decrease along with intrinsic changes in B cells resulting in age-associated effects.22 Moreover, older adults generate tenfold fewer antibody secreting cells relative to young individuals on antigenic stimulation.23
Vaccine-induced myocarditis cases have been reported mostly after the second dose of mRNA COVID-19 vaccination.24 In our study, 3 myopericarditis cases were after the second mRNA COVID-19 vaccination. Acute pericarditis cases (10 patients) developed after the first dose of mRNA vaccination. The reason why myocarditis developed after the second dose of the vaccination was unknown.
There are gender differences in immune responses to pathogens (including to SARS-CoV-2) and vaccines toxicity.25 In general, women generate stronger humoral and cell-mediated immune responses to antigenic stimulation, vaccination and infections than men.26 However, adverse reactions to vaccines may be more prevalent among women when compared to men.27 However, the BioNTech mRNA vaccine results have been published, but do not present data on discontinuation after the first and second dose or adverse reaction data by gender.28
In diagnosis, cardiac involvement should be questioned in the younger population with chest pain after vaccination.29 The evidence of high troponin levels, ECG changes, arrhythmia, and hemodynamic instability after COVID-19 vaccination require hospitalization.
For treatment purposes, according to published case reports, nonsteroidal anti-inflammatory drugs, steroids, and colchicine were used for management of some of the patients with myocarditis after COVID-19 vaccination. Contrary to what is believed, we gave the patients acetylsalicylic acid in addition nonsteroidal anti-inflammatory drugs and the patients got better in early terms.
In heart involvement cases, the idea that the second dose is important is a mystery for clinicians and patients. If vaccine-induced acute pericarditis/myocarditis has developed after the first dose, the second dose should be delayed.30 It has been accepted that the second dose could be reconsidered on the resolution of symptoms, signs, and findings, and cardiac involvement severity.31 Because of abnormal oxidative stress test associated with vaccine-induced myopericarditis, we have suggested that if vaccine-induced myopericarditis develops, the second dose must not be performed. The patients with vaccine-induced acute pericarditis should postpone a second dose of vaccination.
Limitations
Troponin I, D-dimer, hsCRP, and oxidative stress tests were the only markers investigated in this study. This limitation did not affect the results. There is no cardiac biopsy procedure. Furthermore, the study population was small because the study was conducted in a single center. Moreover, mRNA vaccination in Turkey started later than United States and other European countries.
Conclusion
Vaccine-induced myopericarditis cases are associated with oxidative stress test abnormality (abnormal NO, OSI levels). Such a situation has indicated inflammatory and procoagulant state. However, there is no relationship between NO levels and other oxidative stress tests in vaccine-induced acute pericarditis. We think that vaccine-induced pericarditis and myopericarditis could have different pathogenesis. This could be a compelling reason to reconsider the second dose of vaccination for vaccine-induced cardiac involvement.
Abbreviations
TAS, total antioxidant status (mmol Trolox Eq/L); TOS, total oxidant status (mmol H2O2 Eq/L); OSI, oxidative stress index (TOS/TAS); PON, Paraoxonase (U/L); NO, nitric oxide (μmol/L).
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990–2018. Vaccine. 2021;39:839–845. doi: 10.1016/j.vaccine.2020.12.046 [DOI] [PubMed] [Google Scholar]
- 2.Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148(3). doi: 10.1542/peds.2021-052478 [DOI] [PubMed] [Google Scholar]
- 3.Abu Mouch S, Roguin A, Hellou E, et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39(29):3790–3793. doi: 10.1016/j.vaccine.2021.05.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muthukumar A, Narasimhan M, Li QZ, et al. In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine. Circulation. 2021;144(6):487–498. doi: 10.1161/CIRCULATIONAHA.121.056038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP). Coronavirus disease 2019 (COVID-19) vaccines; July 6, 2021.
- 6.Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–175. doi: 10.1016/j.immuni.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 7.Caso F, Costa L, Ruscitti P, et al. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524. doi: 10.1016/j.autrev.2020.102524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uetrecht J, Naisbitt DJ. Idiosyncratic adverse drug reactions: current concepts. Pharmacol Rev. 2013;65(2):779–808. doi: 10.1124/pr.113.007450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saricam E, Dursun AD, Sarıyıldız GT, et al. Laboratory and imaging evaluation of cardiac involvement in patients with post-acute COVID-19. Int J Gen Med. 2021;14:4977–4985. doi: 10.2147/IJGM.S321156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020;55(6):105982. doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect Dis. 2020;6(7):1558–1562. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- 12.Kilic Y, Ozer A, Tatar T, et al. Effect of picroside II on hind limb ischemia reperfusion injury in rats. Drug Des Devel Ther. 2017;11:1917–1925. doi: 10.2147/DDDT.S132401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kucuk A, Polat Y, Kilicarslan A, et al. Irisin protects against hind limb ischemia reperfusion injury. Drug Des Devel Ther. 2021;15:361–368. doi: 10.2147/DDDT.S279318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Saricam E, Saglam Y, Hazirolan T. Clinical evaluation of myocardial involvement in acute myopericarditis in young adults. BMC Cardiovasc Disord. 2017;17(1):129. doi: 10.1186/s12872-017-0564-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nel AE, Miller JF. Nano-enabled COVID-19 vaccines: meeting the challenges of durable antibody plus cellular immunity and immune escape. ACS Nano. 2021;15(4):5793–5818. doi: 10.1021/acsnano.1c01845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khurana A, Allawadhi P, Khurana I, et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021;38:101142. doi: 10.1016/j.nantod.2021.101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayindir M, Bayindir EE. Synergic viral-bacterial co-infection in catalase deficient COVID-19 patients causes suppressed innate immunity and lung damages due to detrimental elevation of hydrogen peroxide concentration. SSRN Electron J. 2020. doi: 10.2139/ssrn.3648292 [DOI] [Google Scholar]
- 22.Frasca D, Blomberg BB. Effects of aging on B cell function. Curr Opin Immunol. 2009;21(4):425–430. doi: 10.1016/j.coi.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol. 2012;24(5):342–349. doi: 10.1016/j.smim.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 24.Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202. doi: 10.1001/jamacardio.2021.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan R, Klein SL. The intersection of sex and gender in the treatment of influenza. Curr Opin Virol. 2019;35:35–41. doi: 10.1016/j.coviro.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engler RJM. Half- vs full-dose trivalent inactivated influenza vaccine (2004–2005): age, dose, and sex effects on immune responses. Arch Intern Med. 2008;168(22):2405. doi: 10.1001/archinternmed.2008.513 [DOI] [PubMed] [Google Scholar]
- 27.CDCMMWR. Allergic reactions including anaphylaxis after receipt of the first dose of PfizerBioNTech COVID-19 vaccine — United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saricam E, Saglam Y. Potentially missed acute pericarditis: atypical pericarditis. Am J Emerg Med. 2016;34(12):2451–2453. doi: 10.1016/j.ajem.2016.09.020 [DOI] [PubMed] [Google Scholar]
- 30.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33(36):4398–4405. doi: 10.1016/j.vaccine.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Clinical considerations; myocarditis and pericarditis after receipt of mRNA COVID-19 vaccines among adolescents and young adults; May 28, 2021. Available from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html. Accessed July 6, 2021.
- 32.Akinosoglou K, Tzivaki, I, Marangos M. Covid-19 vaccine and autoimmunity: Awakening the sleeping dragon. Clin Immunol. 2021;226:108721. doi: 10.1016/j.clim.2021.10872122 [DOI] [PMC free article] [PubMed]


