Figure 2.
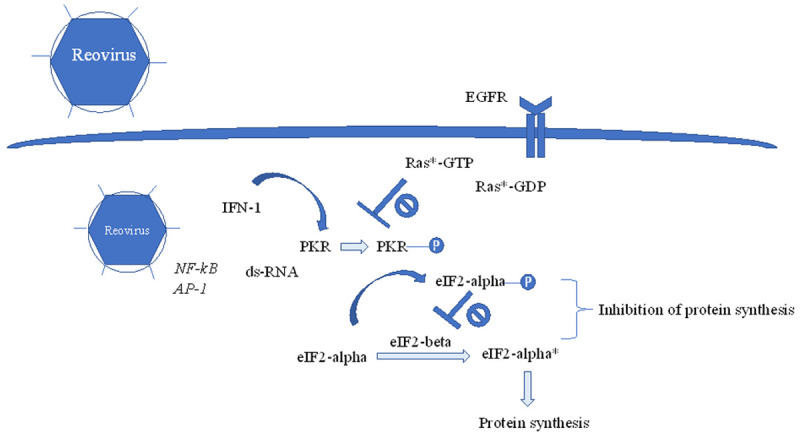
Reovirus-mediated oncolysis is negatively regulated by PKR. Upon reovirus infection, transcription factors such as NF-κB and AP-1 are activated, which leads to increase in interferon-1. Increase in interferon-1 leads to upregulation and autophosphorylation of PKR, which phosphorylates eIF2-alpha. Phosphorylated eIF2-alpha binds to eiF2-beta with high affinity, which in its free form, converts eiF2-alpha from its inactive to active state, leading to protein translation. eiF2-beta, when bound to phosphorylated eIF2-alpha, prevents recycling of active eiF2-alpha as a substrate, thereby blocking protein synthesis. In RAS-mutant cells, phosphorylation of PKR is inhibited, leading to uninhibited protein synthesis and reovirus replication.
