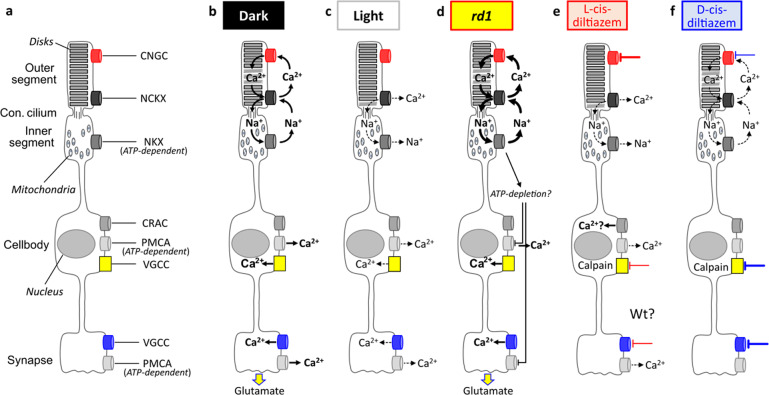
Fig. 7. Schematic representation of photoreceptor Ca2+ flux under different experimental conditions.
a The phototransduction cascade is compartmentalised to the photoreceptor outer segments, which harbour cyclic nucleotide-gated channel (CNGC) and Na+/Ca2+/K+ exchanger (NCKX). The connecting cilium links outer to inner segment, which holds almost all mitochondria and the ATP-driven Na+/K+ exchanger (NKX). The cell body harbours the nucleus as well as Ca2+-release activated channel (CRAC), plasma membrane Ca2+-ATPase (PMCA), and voltage-gated Ca2+ channels (VGCC). PMCA and VGCC are also found in the synapse. b In the dark, the flux of Na+ and Ca2+ ions across the photoreceptor membrane (i.e., the dark current) keeps the cell in a continuously depolarised state. The Ca2+ ions enter the outer segment via CNGC and exits via NCKX. The Na+ gradient needed to drive NCKX is maintained by the ATP-dependent NKX in the inner segment. At the same time, in the photoreceptor cell body and synapse, VGCC allows for Ca2+ influx, mediating synaptic glutamate release. In the cell body and synapse, Ca2+ is extruded by the ATP-dependent PMCA. c In light, CNGC closes, while Ca2+ continues to exit the cell via NCKX, leading to photoreceptor hyperpolarization. This in turn closes VGCC, ending synaptic glutamate release. d In rd1 photoreceptors, high cGMP continuously opens CNGC, representing a situation of “constant darkness”. Excessive NKX activity in rd1 may cause a depletion of ATP, preventing Ca2+ extrusion via PMCA. e L-cis-diltiazem (red lines) blocks predominantly CNGC, with an additional block on VGCC at high concentrations. This resembles a situation of “constant light” and may cause a depletion of intracellular Ca2+ and secondary Ca2+ influx via activation of CRAC. f D-cis-diltiazem (blue lines) inhibits predominantly VGCC, with a partial block on CNGC at high concentrations.