Abstract
Background
Posterolateral corner (PLC) injuries of the knee are often overlooked for its complex anatomy, and frequent association with cruciate ligament injuries. Overlooked injuries lead to reconstruction failure of cruciate ligaments, chronic knee pain and early arthritic changes. Many reconstruction methods are described, but the best treatment still remains elusive. In this study, we have treated grade-III PLC injuries by the ‘anatomic LaPrade’ technique and the ‘fibula-based Modified Larson’ technique, and evaluated their outcomes. Our hypothesis was that both the groups will have similar improvements after surgery.
Methods
An open-label prospective comparative study was done with a total of 28 patients from August 2013 to July 2019. Patients were treated alternatively by LaPrade or Modified Larson technique using hamstring autografts. Follow-up visits were done at sixth week and subsequently at 3, 6, 12, 18 and 24 months postoperatively. Outcomes were measured by Dial Test, side-to-side difference in lateral opening on varus stress radiographs, Lysholm score and IKDC subjective score.
Results
During analysis, we considered 25 patients only as three patients were lost to follow-up. Both the groups had comparable improvements in rotational stability, lateral opening on varus stress, Lysholm score and IKDC subjective score.
Conclusion
Both LaPrade and Modified Larson technique showed good clinical results in restoring varus and rotational stability of knee in grade-III posterolateral corner injury of the knee.
Level of evidence
II (prospective, comparative study)
Keywords: Posterolateral corner of knee, PLC reconstruction, LaPrade versus modified Larson
Introduction
In recent years with increase in road-traffic accidents and sports injuries, the incidence of knee joint ligament injuries is increasing; and with improving infra-structure and surgical skills, more and more ligament reconstructions are also being done. With increase in number of reconstructive surgeries in relation to sports injuries around the knee, more and more cases of reconstructive failures are being diagnosed. In search of the causes of these failures, the “darker side of knee”, i.e., the Posterolateral corner (PLC) of knee came into light as one important cause. [1, 2] Injury to PLC of knee is uncommon accounting for 16% of knee ligament injuries [2] and it is frequently related to high energy trauma. Isolated injuries are even rarer accounting for 2% of injuries [3, 4]. PLC provides both static and dynamic stability to knee. Injuries to these structures are often overlooked for their complex anatomy, and frequent association with cruciate ligament injuries. Overlooked injuries lead to reconstruction failure of cruciate ligaments, persistent posterolateral instability with varus thrust gait, chronic knee pain and early arthritic changes [5–7]. Many reconstruction methods are described in the literature, ranging from augmentations and non-anatomic reconstructions like biceps tenodesis, to more anatomic ones like the combined tibia- and fibula-based anatomic reconstruction as described by LaPrade et al. [8–12] and the femur- and fibula-based anatomic reconstruction such as Modified Larson technique; but the best treatment still remains elusive. The LaPrade anatomic technique aims at reconstruction of the three main stabilizers of the PLC of knee, namely the lateral collateral ligament (LCL), popliteus tendon (PT), and popliteofibular ligament (PFL); whereas, only LCL and PFL are reconstructed in the modified Larson technique. In this study, we have treated grade-III PLC injuries by the ‘anatomic LaPrade’ technique and the ‘fibula-based Modified Larson’ technique, and evaluated their outcomes. Our hypothesis was that both the groups will have similar improvements after surgery.
Methods
Our study was conducted from August 2013 to July 2019 after approval by the institutional ethical committee. It was an open-label, prospective, non-randomized, comparative study. Inclusion criteria were: symptomatic grade-III PLC injury with or without other ligament injuries of the knee. All the patients were explained about their inclusion in the study and informed consents were taken.
Exclusion criteria were: hyperlaxity symptoms, bilateral knee injuries, elderly patients with low functional demand, associated major fractures around same/opposite knee, ligament avulsion fractures, significant meniscus injury requiring balancing or repair and chronic cases with varus malalignment who required high tibial osteotomy to correct limb alignment before PLC reconstruction.
Only 28 patients fulfilled our inclusion and exclusion criteria. Allocation into ‘LaPrade group’ or ‘Modified Larson group’ was done alternatively (i.e., every even number patient underwent PLC reconstruction using Modified Larson’s technique). Thus, the groups were allocated 14 patients each.
All the patients were examined clinically using standard tests for knee instability and associated injuries specially Varus–Valgus stress test, anterior and posterior Drawer test, posterolateral Drawer test, dial test, external rotation recurvatum test and their findings were recorded.
The knees were further examined carefully for any signs of bony varus malalignment and varus thrust during stance phase of ambulation. This was further clarified with standing full length anteroposterior radiograph or scanogram.
All the knees were evaluated with standard radiograph (AP and lateral view), Varus–Valgus stress radiograph and MRI (Fig. 1); and findings were recorded accordingly.
Fig. 1.
19 years old male patient with ACL and PLC injury in left knee. a MR images confirming the diagnosis (red arrow), b, c clinical, and d radiological images at final follow-up after PLC reconstruction by modified Larson technique
In the operation theater, after examination under anesthesia (EUA), the patients were positioned supine with the affected knee and leg hanging from the edge of the table. Under tourniquet control, diagnostic arthroscopy was done to look for any menisco-ligamentous injury; cartilage injury (especially medial compartment) and presence of ‘lateral drive through’ sign, i.e., greater than 1 cm of lateral joint line opening to a varus stress applied to the joint at the time of arthroscopic evaluation (Fig. 2).
Fig. 2.
Intraoperative images showing: a cartilage damage at medial compartment of left knee, b positive lateral drive through sign on diagnostic arthroscopy, c PLC reconstruction by LaPrade anatomical technique in right knee. Note the fibular and tibial tunnels marked by suture loops after dissection and retraction of the common peroneal nerve and guide wires for femoral tunnels of LCL and PT placed about 18 mm apart, d PLC reconstruction by modified Larson technique in left knee. Fibular tunnel marked by suture loop in similar way
After confirmation of the preoperative planning by EUA and diagnostic arthroscopy, regarding the ligaments to be reconstructed, grafts were harvested as per requirement. We used autografts for our procedures: ipsilateral hamstrings for PLC reconstructions (a single-stranded semitendinosus graft with average length of 20–24 cm for reconstruction of both LCL and PFL in modified Larson group; whereas, a single-stranded semitendinosus graft for LCL and PFL, and double stranded gracillis for PT reconstruction in the LaPrade group); and contralateral hamstrings for reconstruction of associated ACL/PCL injuries. In one patient with combined PLC, PCL and ACL injuries, contralateral hamstrings were used for PCL reconstruction and ipsilateral quadriceps for ACL. One patient with PLC and ACL injury refused graft harvest from opposite limb, where ipsilateral bone patellar tendon bone graft was used for ACL reconstruction.
Our sequence of reconstructions was single-bundle PCL first (if present), followed by PLC and single-bundle ACL simultaneously. A gently curved, L-shaped incision was made on the lateral aspect of the knee starting proximal to the lateral femoral condyle and continuing distally to mid-point between the fibular head and Gerdy’s tubercle. After dissection through subcutaneous layers, three fascial incisions described by LaPrade et al. [13] were used for both the groups. The first posterior-most fascial incision was made posterior and parallel to the biceps femoris tendon to expose and mobilize the common peroneal nerve (CPN). This was protected throughout the procedure. Through this incision, the posterior aspect of the proximal fibula and the proximal tibio-fibular joint could be exposed. Then, the fibular tunnel was drilled at the LCL attachment site from anterolateral to posteromedial direction first with a Beath pin followed by a 6- or 7-mm reamer. A looped passing suture was then placed through this tunnel for future use.
A second fascial incision was made in between the biceps femoris tendon and the iliotibial band. Developing this interval would expose the posterior aspect of the lateral tibial plateau for tibial tunnel preparation for attachment of the PFL and PT. Through this interval we could identify the LCL, and trace to its femoral and fibular attachment sites. However, this fascial plane was not compulsory for the modified Larson technique. Then, the 8- or 9-mm diameter tibial tunnel was prepared in the LaPrade group, with the help of a PCL femoral tunnel-aiming device, 10 mm distal and parallel to the tibial articular surface, starting at the flat spot just distal and medial to the Gerdy’s tubercle, directing posteriorly to the popliteal sulcus of tibia which marks the location of the musculotendinous junction of the popliteus. A looped passing suture was then placed through this tunnel for future use.
The third fascial incision was made through the iliotibial band, in line with its fibers, centered over the lateral femoral epicondyle. The femoral attachments of the LCL (slightly proximal and posterior to the lateral epicondyle) and PT (at anterior aspect of the popliteal groove) were identified; and two eyelet-tipped guide pins were introduced approximately 18 mm apart from lateral to medial, directing superiorly (to avoid convergence with femoral tunnel of PCL) and anteriorly (to avoid convergence with femoral tunnel of ACL). A 7- or 8-mm femoral tunnel was then reamed over each guide pin to a depth of 25–30 mm. The medial cortices were drilled with a 4.5 mm cortical breaker over the guide pins to facilitate graft passage.
The grafts were then passed through the ligaments’ normal anatomical courses, which were developed bluntly with the use of a curved hemostat. The sequences of graft fixations were slightly different in the two groups, as described by the original authors of the two techniques [13, 14]. In the LaPrade group, grafts were fixed to the femoral tunnels of LCL and PT first, followed by the fibular tunnel with the knee at 30° of flexion and neutral rotation and a slight valgus stress, and lastly the tibial tunnels at 60° of flexion and 5° of internal rotation of the knee. In the modified Larson group, grafts were first fixed to the fibular tunnels, followed by the femoral tunnels in 30° of flexion of the knee with slight internal rotation and valgus stress. We used metal or bio-absorbable interference screws (7 mm for fibular tunnel, 8 mm for femoral tunnels and 9 mm for tibial tunnel) for securing the grafts during PLC reconstruction (Depuy or Smith–Nephew).
Conscious efforts were made to keep the tourniquet time within 2 h. If more time was required, a 15-min interval was allowed before re-inflating the tourniquet.
Postoperative Management
A common rehabilitation protocol was followed for both the groups of patients. Postoperatively, they were given a long knee brace for temporary immobilization. No prophylaxis was given for deep vein thrombosis.
Isometric quadriceps exercise was started from second day onwards along with knee range of motion (ROM) exercises as tolerated. They were discharged from the hospital on third day; and were supervised once a week on outpatient basis to achieve a minimum target ROM of 90° by 3 weeks, thereafter, as much and as early as possible.
All patients were put on gradual non-weight bearing mobilization using two axillary crutches for the initial 6 weeks. Then partial weight bearing was allowed, progressing gradually to full weight bearing by 3 months postoperatively.
Quadriceps and hamstring strengthening exercises were introduced sixth week onwards. Jogging on plain surface was started after 4 months.
All day-to-day activities were allowed by 6 months. Return to sports activities were permitted only after 9 months when normal strength, stability, and knee range of motion comparable to the contralateral side have been achieved.
Follow-Up
All patients were reviewed at the outpatient department at 6th week, and 3, 6, 12, 18 and 24 months after surgery and yearly thereafter. At each visit, patients were assessed clinically, along with functional assessment with IKDC Subjective score and Lysholm knee score.
Statistical Analysis
During analysis of data, only those patients were considered who completed at least 2-year follow-up after surgery. Statistical analysis was conducted by an independent statistician who was not associated with the surgical team. All the data were tested for ‘Normality’ by Kolmogorov–Smirnov test. The normally distributed data like age and operating time were compared for significance using unpaired t test. The differences between the preoperative and final follow-up variables of side-to-side difference on varus stress radiograph, Lysholm score and IKDC subjective score were calculated; and were compared between the two groups for statistical significance using ‘independent samples Mann–Whitney U test’ (SPSS software version 16). P value < 0.05 was considered significant.
Results
During analysis of results, only 25 patients were considered as three patients (two from ‘LaPrade group’ and one from ‘Modified Larson group’) were lost to follow-up. Average age of patient at the time of surgery was 27.44 years (18–38 years). Most of the patients were male, only two being female patients. The average follow-up period was 31.3 months (24–47 months).
Seven patients had isolated PLC injury, while eight had associated ACL and nine had associated PCL injury. One patient had both ACL and PCL injury additionally (Table 1). Twelve of them were treated by LaPrade technique, while the rest by Modified Larson technique.
Table 1.
Comparison of ligaments injured
| LaPrade group | Modified Larson group | Total | |
|---|---|---|---|
| Isolated PLC | 3 | 4 | 7 (28%) |
| PLC + PCL | 3 | 6 | 9 (36%) |
| PLC + ACL | 5 | 3 | 8 (32%) |
| PLC + PCL + ACL | 1 | 0 | 1 (4%) |
| Total | 12 | 13 | 25 |
The preoperative mean side-to-side difference in lateral opening seen on varus stress radiograph in the LaPrade group was 5.50 ± 0.90 mm (4–7 mm); and at final follow-up, it was 0.50 ± 0.52 mm (0–1 mm). In the Modified Larson group, before surgery it was 5.31 ± 0.85 mm (4–7 mm); and at last follow-up it was 0.77 ± 0.60 mm (0–2 mm) (Table 2). Postoperatively, all the patients had negative dial test, which were positive before surgery.
Table 2.
Details of preoperative and final follow-up data
| LaPrade group | Modified Larson group | ||
|---|---|---|---|
| Side-to-side difference seen on varus stress radiograph (in mm) | Preoperative | 5.50 ± 0.90 | 5.31 ± 0.85 |
| at final follow-up | 0.50 ± 0.52 | 0.77 ± 0.60 | |
| Lysholm scores | Preoperative | 36.67 ± 6.24 | 37.85 ± 5.05 |
| at final follow-up | 89.00 ± 7.76 | 91.54 ± 3.93 | |
| IKDC subjective scores | Preoperative | 36.29 ± 5.89 | 39.69 ± 4.19 |
| At final follow-up | 79.70 ± 8.65 | 83.31 ± 6.05 |
The average Lysholm score in the LaPrade group improved from 36.67 ± 6.24 preoperatively to 89.00 ± 7.76 at last follow-up, and in the Modified Larson group from 37.85 ± 5.05 preoperatively to 91.54 ± 3.93 at last follow-up. Preoperatively, the IKDC subjective knee score was 36.29 ± 5.89 in the LaPrade group and 39.69 ± 4.19 in the Modified Larson group, and became 79.70 ± 8.65 and 83.31 ± 6.05, respectively, at final follow-up (Table 2).
The differences between the preoperative and final follow-up data are mentioned in Table 3. No statistically significant difference was noted between the two groups in operating time and hospital stay (Table 4).
Table 3.
Comparison of difference in preoperative and final follow-up data between groups
| LaPrade group | Modified Larson group | P value | |
|---|---|---|---|
| Side-to-side difference seen on varus stress radiograph (in mm) | 5.00 ± 0.74 | 4.54 ± 0.52 | 0.15 |
| Lysholm scores | 52.33 ± 6.67 | 53.69 ± 3.09 | 0.81 |
| IKDC subjective scores | 43.41 ± 8.07 | 43.62 ± 3.03 | 0.41 |
Table 4.
Comparison of other relevant data between groups
| LaPrade group | Modified Larson group | P value | Statistical method | |
|---|---|---|---|---|
| Age (years) | 27.33 ± 7.14 | 27.54 ± 5.85 | 0.94 | Unpaired t test |
| Operating time (min) | 127.08 ± 26.58 | 115.77 ± 12.39 | 0.18 | Unpaired t test |
| Hospital stay (days) | 5.08 ± 0.52 | 4.77 ± 0.44 | 0.25 | Mann–Whitney U test |
We had three complications in two patients in our study: stiffness with surgical site infection in one patient, and stiffness with heterotopic ossification in the other. Both of them belonged to the LaPrade group.
Discussion
Many studies have reported several techniques of PLC reconstruction with their biomechanical as well as clinical results, but the best surgical technique remains a matter of debate [8–12]. Many surgical techniques including repairs, augmentations and non-anatomic reconstructions like biceps tenodesis were done previously, but abandoned nowadays in view of poor functional outcome and high failure rates [1, 12, 15, 16].
The LCL and the PFL are the two main static stabilizers, whereas the popliteus muscle–tendon unit functions as the dynamic stabilizer of the PLC of knee. In 2004, LaPrade et al. [13, 17, 18] described an anatomic technique of the PLC reconstruction using the native attachments of the LCL, PT and PFL. This was one of the earliest descriptions of a surgical option to recreate the anatomy of the three main static stabilizers of the PLC. On the contrary, the fibula-based modified Larson technique was relatively simple, but still useful with reconstruction of the LCL and PFL only [2, 11, 14, 19], although the PFL attachment was slightly modified from its anatomical attachment. The modified Larson technique had mainly two modifications over the original Larson technique: (1) two separate femoral tunnels for attachments of the LCL and PFL rather than a single isometric femoral tunnel, and (2) slightly oblique orientation of the fibular tunnel from anterolateral to posteromedial direction rather than anterior to posterior. These two modifications allow recreation of more anatomic attachments of the native LCL and PFL, along with reduction in the incidence of fibular head fracture [14, 19]. In biomechanical studies, no statistically significant difference has been found in the ability of the above two reconstruction methods, the LaPrade and the modified Larson, to control posterolateral instability [20]. Therefore, the aim of this study was to compare these two currently used techniques for PLC reconstruction on clinical grounds to evaluate how closely each technique restores varus and rotational stability to normalcy, and to compare their clinical outcomes.
The collective incidence of isolated PLC injury of the knee in our series was 28% (Table 1) which was relatively high as per literature, but comparable with the original series by LaPrade et al. [17]. Associated injury to the cruciate ligaments or menisci adversely affects the functional outcome of PLC reconstruction of the knee [6, 16]. Chronic PLC injury of the knee leads to abnormal varus thrust gait, significant changes in articular contact pressure at the medial compartment and progressive secondary osteoarthritis. That is why medial compartment cartilage damage on diagnostic arthroscopy is seen as indirect evidence of PLC injury of the knee. Another confirmatory evidence of significant PLC injury of the knee is the presence of ‘lateral drive through’ sign, i.e., greater than 1 cm of lateral joint line opening to a varus stress applied to the joint at the time of diagnostic arthroscopy [9]. LaPrade et al. [21] have shown in 2008, in a cadaveric study, an approximately 2.7 mm increase in lateral opening on varus stress radiographs after isolated LCL injury compared to 4.0 mm increase in cases with grade-III PLC injury. These findings are comparable with those of our study, where varus and rotational instability were well controlled in both the groups (Tables 2, 3) with no statistically significant difference, with only one patient from ‘Modified Larson group’ showing a side-to-side difference in lateral opening of 2 mm compared to the intact knee at final follow-up [22, 23].
We found significant improvements in Lysholm scores and IKDC subjective scores after surgery, i.e., from preoperative to final follow-up scores in both the groups. But when we compared the differences [23] in the preoperative and final follow-up scores between the two groups, they were not statistically significant (Table 3). Therefore, we did not find any clinical evidence to support the use of the more complex reconstruction method similar to some other biomechanical studies [20].
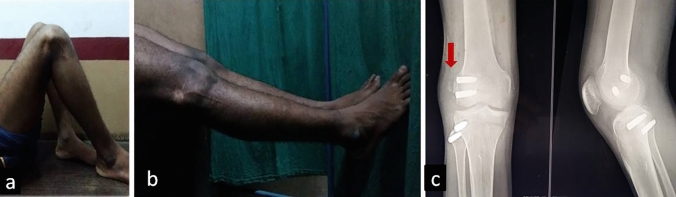
We faced three complications in two patients: first one was an immediate complication in the form of infection at surgical site that was managed promptly with debridement and antibiotics; the second one was a late complication in the form of heterotopic ossification (Fig. 3), which was treated by non-operative means initially, but required excision after maturation. It is believed that reaming for the graft tunnels particularly femoral tunnels for PLC reconstruction contributes to the process [24]. Therefore, thorough lavage with normal saline should be given to remove the bone debris to prevent this complication. Both these patients developed the third complication, i.e., residual stiffness, for which they refused any further treatment. CPN injury is an important complication that needs to be avoided when one is performing lateral knee procedures. In our series, we exposed the nerve early in the procedure where the nerve passes the fibular neck. This enables nerve protection by visual confirmation during the drilling procedures that most likely could cause nerve injury. In our series no nerve injuries were observed.
Fig. 3.
An 18-year-old male patient with isolated PLC injury treated by LaPrade anatomical reconstruction developing complications. a, b clinical images of knee stiffness (note the swelling over lateral femoral condyle), c heterotopic ossification (red arrow)
Similar to any other complex surgical procedures, PLC reconstruction is also associated with significant learning curve [25]. Our experience is no different in this regard as the operating time decreased gradually in the later part of our series compared to the initial few cases.
In recent years newer arthroscopic procedures for anatomical reconstruction of the PLC have been described that could have the potential to replace open procedures in posterolateral corner surgery of the knee in the future [25–27]. However, their usefulness with regard to biomechanical stability tests and clinical implications are yet to be established.
Only a few clinical studies have presented outcomes after PLC reconstruction comparing the two techniques that have stood the test of time so far. The authors believe that this study with a minimum of 2-year follow-up should be able to reflect some light on this ‘darker side of the knee’. However, our study is limited by the fact that the study population is small. Randomized controlled trials with large sample size and longer follow-ups are required to further illuminate and standardize the treatment protocols of PLC injuries in future [28].
To conclude, in institutions like ours where we are forced to rely on autografts only, owing to the lack of tissue banks and financial constraints, Modified Larson technique seems to be a better treatment option for PLC reconstruction, specially with multiple ligament injuries, in view of less graft requirement and comparable clinical outcome.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the institutional ethical committee.
Ethical Standard Statement
This article does not contain any studies with human or animal subjects performed by the any of the author
Informed Consent
For this type of study informed consent is not required.
Consent for Participation and Publication
All the patients were explained about their inclusion in the study and that the data will be used for publication in future; and informed written consents were taken.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amit Sharma, Email: amit8707@gmail.com.
Partha Saha, Email: partha.orthoatnrs@gmail.com.
Utpal Bandyopadhyay, Email: utpal0107@gmail.com.
References
- 1.Noyes FR, Barber-Westin SD, Albright JC. An analysis of the causes of failure in 57 consecutive posterolateral operative procedures. American Journal of Sports Medicine. 2006;34:1419–1430. doi: 10.1177/0363546506287743. [DOI] [PubMed] [Google Scholar]
- 2.Camarda L, Condello V, Madonna V, Cortese F, D’Arienzo M, Zorzi C. Results of isolated posterolateral corner reconstruction. Journal of Orthopaedics and Traumatology. 2010;11:73–79. doi: 10.1007/s10195-010-0088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaPrade RF, Wentorf FA, Fritts H, Gundry C, Hightower CD. A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy. 2007;23:1341–1347. doi: 10.1016/j.arthro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 4.DeLee JC, Riley MB, Rockwood CA., Jr Acute posterolateral rotatory instability of the knee. American Journal of Sports Medicine. 1983;11:199–207. doi: 10.1177/036354658301100403. [DOI] [PubMed] [Google Scholar]
- 5.Wentorf FA, LaPrade RF, Lewis JL, Resig S. The influence of the integrity of posterolateral structures on tibiofemoral orientation when an anterior cruciate ligament graft is tensioned. American Journal of Sports Medicine. 2002;30:796–799. doi: 10.1177/03635465020300060701. [DOI] [PubMed] [Google Scholar]
- 6.Dean RS, LaPrade RF. ACL and posterolateral corner injuries. Current Reviews in Musculoskeletal Medicine. 2020;13(1):123–132. doi: 10.1007/s12178-019-09581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaPrade RF, Muench C, Wentorf F, Lewis JL. The effect of injury to the posterolateral structures of the knee on force in a posterior cruciate ligament graft: a biomechanical study. American Journal of Sports Medicine. 2002;30(2):233–238. doi: 10.1177/03635465020300021501. [DOI] [PubMed] [Google Scholar]
- 8.Covey DC. Injuries of the posterolateral corner of the knee. Journal of Bone and Joint Surgery. 2001;83:106–118. doi: 10.2106/00004623-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 9.LaPrade RF, Wentorf F. Diagnosis and treatment of posterolateral knee injuries. Clinical Orthopaedics and Related Research. 2002;402:110–121. doi: 10.1097/00003086-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Veltri DM, Warren RF. Operative treatment of posterolateral instability of the knee. Clinics in Sports Medicine. 1994;13:615–627. doi: 10.1016/S0278-5919(20)30313-6. [DOI] [PubMed] [Google Scholar]
- 11.Niki Y, Matsumoto H, Otani T, Enomoto H, Toyama Y, Suda Y. A modified Larson’s method of posterolateral corner reconstruction of the knee reproducing the physiological tensioning pattern of the lateral collateral and popliteofibular ligaments. Sports Medicine, Arthroscopy, Rehabilitation, Therapy and Technology. 2012;4:21. doi: 10.1186/1758-2555-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon KH, Lee SH, Park SY, Park SE, Tak DH. Comparison of anatomic posterolateral knee reconstruction using 2 different popliteofibular ligament techniques. American Journal of Sports Medicine. 2016;44(4):916–921. doi: 10.1177/0363546515623966. [DOI] [PubMed] [Google Scholar]
- 13.LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. American Journal of Sports Medicine. 2004;32:1405–1414. doi: 10.1177/0363546503262687. [DOI] [PubMed] [Google Scholar]
- 14.Arciero RA. Anatomic posterolateral corner knee recostruction. Arthroscopy. 2005;21:1147. doi: 10.1016/j.arthro.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 15.McGuire DA, Wolchok JC. Posterolateral corner reconstruction. Arthroscopy. 2003;19:790–793. doi: 10.1016/S0749-8063(03)00387-6. [DOI] [PubMed] [Google Scholar]
- 16.Zorzi C, Alam M, Iacono V, Madonna V, Rosa D, Maffulli N. Combined PCL and PLC reconstruction in chronic posterolateral instability. Knee Surgery, Sports Traumatology, Arthroscopy. 2013;21:1036–1042. doi: 10.1007/s00167-011-1771-y. [DOI] [PubMed] [Google Scholar]
- 17.LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L. Outcomes of an anatomic posterolateral knee reconstruction. Journal of Bone and Joint Surgery. 2010;92:16–22. doi: 10.2106/JBJS.I.00474. [DOI] [PubMed] [Google Scholar]
- 18.Cruz RS, Mitchell JJ, Dean CS, Chahla J, Moatshe G, LaPrade RF. Anatomic posterolateral corner reconstruction. Arthroscopy Techniques. 2016;5(3):563–572. doi: 10.1016/j.eats.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanelli GC, Larson RV. Practical management of posterolateral instability of the knee. Arthroscopy. 2002;18:1–8. doi: 10.1053/jars.2002.31779. [DOI] [PubMed] [Google Scholar]
- 20.Apsingi S, Nguyen T, Bull AMJ, Unwin A, Deehan DJ, Amis AA. A comparison of modified Larson and ‘anatomic’ posterolateral corner reconstructions in knees with combined PCL and posterolateral corner deficiency. Knee Surgery, Sports Traumatology, Arthroscopy. 2009;17:305–312. doi: 10.1007/s00167-008-0696-6. [DOI] [PubMed] [Google Scholar]
- 21.LaPrade RF, Heikes C, Bakker AJ, Jakobsen RB. The reproducibility and repeatability of varus stress radiographs in the assessment of isolated fibular collateral ligament and grade-III posterolateral knee injuries. An in vitro biomechanical study. Journal of Bone and Joint Surgery. 2008;90(10):2069–2096. doi: 10.2106/JBJS.G.00979. [DOI] [PubMed] [Google Scholar]
- 22.Kim SJ, Kim SG, Lee IS, Han HD, Chung IH, Kim SH, Gorthi V. Effect of physiological posterolateral rotary laxity on early results of posterior cruciate ligament reconstruction with posterolateral Corner reconstruction. Journal of Bone and Joint Surgery. 2013;95:1222–1227. doi: 10.2106/JBJS.L.00861. [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Lee SK, Kim SH, Kim SH, Jung M. Clinical outcomes for reconstruction of the posterolateral corner and posterior cruciate ligament in injuries with mild grade 2 or less posterior translation—comparison with isolated posterolateral corner reconstruction. American Journal of Sports Medicine. 2013;41(7):1613–1620. doi: 10.1177/0363546513485927. [DOI] [PubMed] [Google Scholar]
- 24.Patton WC, Tew WM. Periarticular heterotopic ossification after multiple knee ligament reconstructions. A report of three cases. American Journal of Sports Medicine. 2000;28:398–401. doi: 10.1177/03635465000280032001. [DOI] [PubMed] [Google Scholar]
- 25.Frosch KH, Akoto R, Drenck T, Heitmann M, Pahl C, Preiss A. Arthroscopic popliteus bypass graft for posterolateral instabilities of the —a new surgical technique. Operative Orthopädie und Traumatologie. 2016;28:193–203. doi: 10.1007/s00064-015-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frosch KH, Akoto R, Heitmann M, Enderle E, Giannakos A, Preiss A. Arthroscopic reconstruction of the popliteus complex: accuracy and reproducibility of a new surgical technique. Knee Surgery, Sports Traumatology, Arthroscopy. 2015;23:3114–3120. doi: 10.1007/s00167-014-3000-y. [DOI] [PubMed] [Google Scholar]
- 27.Song G, Zhang H, Zhang J, Li Y, Feng H. Anatomical popliteofibular ligament reconstruction of the knee joints: an all-arthroscopic technique. Knee Surgery, Sports Traumatology, Arthroscopy. 2015;23:2925–2929. doi: 10.1007/s00167-015-3531-x. [DOI] [PubMed] [Google Scholar]
- 28.LaPrade RF, Griffith CJ, Coobs BR, Geeslin AG, Johansen S, Engebretsen L. Improving outcomes for posterolateral knee injuries. Journal of Orthopaedic Research. 2014;32:485–491. doi: 10.1002/jor.22572. [DOI] [PubMed] [Google Scholar]