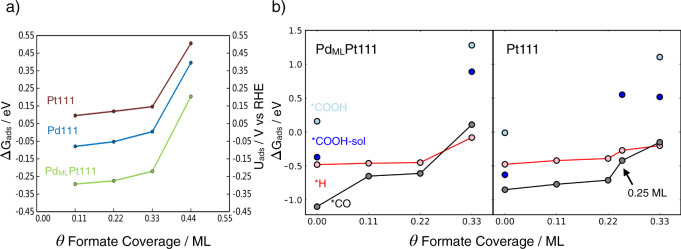
Fig. 5. Adsorption energies and potentials of adsorbates.
a Adsorption/formation energies (left y-axis) and corresponding adsorption potentials (right y-axis) for adsorbed formate from formic acid in solution as a function of coverage, on PdMLPt(111) (green), Pd(111) (blue), and Pt(111) (red). b Free energy of adsorption of *H, *CO, and *COOH corresponding to 1/9 ML at 0 V vs RHE, for PdMLPt(111) and Pt(111), as a function of different coverages of co-adsorbed formate from 0.11 to 0.33 ML also including 0.25 ML for Pt(111) as specified with the arrow, because this is the experimentally observed maximum coverage on Pt(111). Only the low and higher coverages for *COOH are shown (0.11 ML and 0.33 ML), where *COOH-sol (blue) is with solvation and *COOH (light blue) is without. Connecting lines are only intended as a guide for the eye.