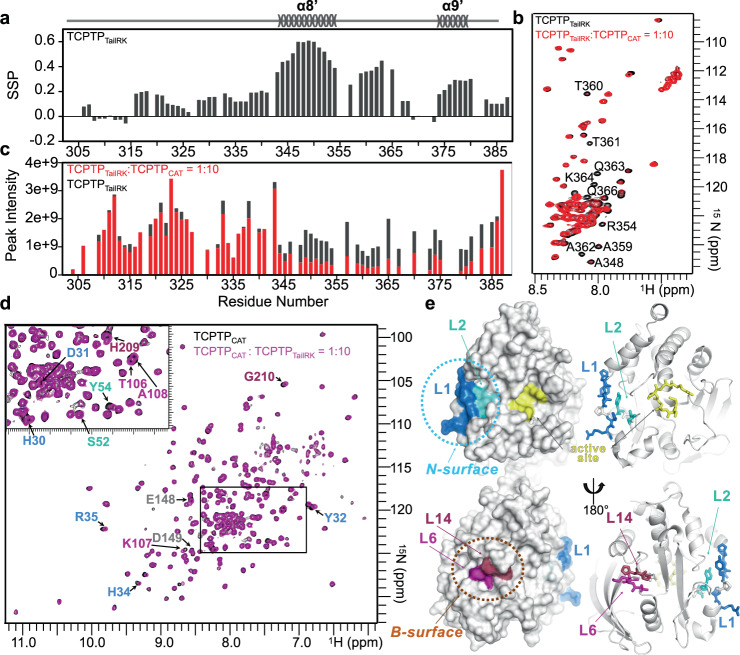
Fig. 3. The TCPTP dynamic C-terminal tail binds the TCPTP catalytic domain.
a SSP vs TCPTPTaiRK residues. SSP highlights transient secondary structure elements in the TCPTPTaiRK C-terminal tail (SSP > 0, α helix; SSP < 0, β strand). b Overlay of the 2D [1H,15N] HQSC spectra of 15N-labeled TCPTPTailRK (black) and TCPTPCAT-bound TCPTPTailRK (red). Residues with significant reductions in cross peak intensity are annotated. c Peak intensity comparison between free TCPTPTailRK (black) and TCPTPCAT-bound TCPTPTailRK (red). d Overlay of the 2D [1H,15N] TROSY spectra of (2H,15N)-labeled TCPTPCAT (black) and TCPTPTailRK-bound TCPTPCAT (magenta). Peaks with chemical shift perturbations (CSPs) are labeled (colors corresponding to residues in L1, L2, L6, L14 as in e). e Residues with CSPs in d are mapped onto the 3D structure of TCPTPCAT (PDB: 1L8K). Residues cluster in two regions, the N-surface (loop L1, blue; L2, cyan) which is to the left edge of the active site (yellow) and the B-surface (L6, magenta; L14, raspberry), which is on the opposite face as the active site.