Fig. 1. Nhp6 protein interacts with C-terminal domains of FACT subunits.
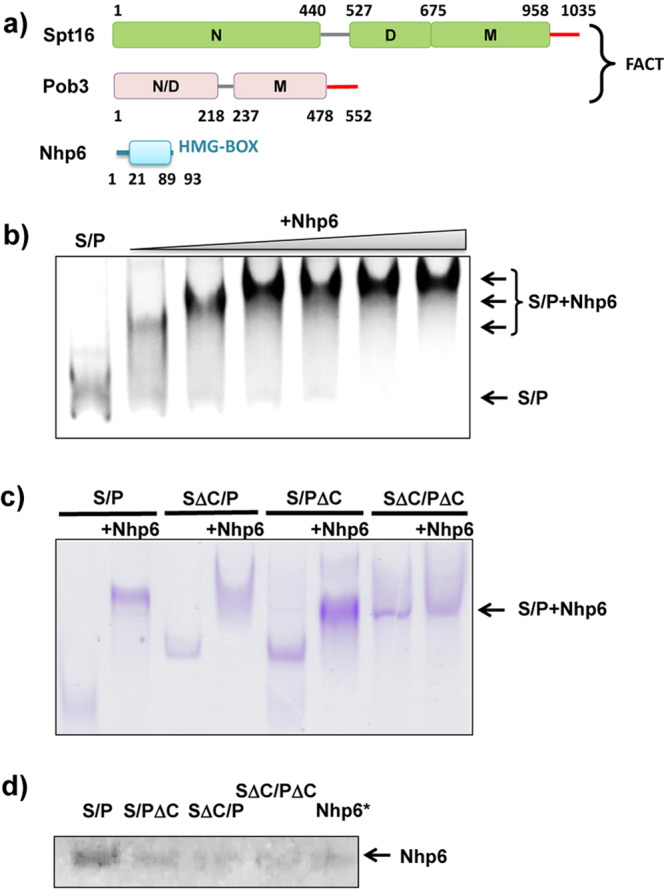
a FACT and Nhp6 domain structures. FACT is a dimer of Spt16 and Pob3 subunits and requires Nhp6 protein for nucleosome unfolding. N N-terminal domain, D dimerization domains, M middle domains, N/D N-terminal/dimerization domain. Negatively charged C-terminal regions of Spt16 and Pob3 are shown in red. b Spt16/Pob3 (S/P, 0.13 µM) was incubated with Nhp6 (0, 0.26 µM, 0.52 µM, 0.78 µM, 1.04 µM, 1.3 µM, or 2.6 µM) and analyzed by native PAGE followed by silver staining. Arrows indicate distinct migration patterns. c Native PAGE analysis of the migration of FACT mutants lacking the C-terminal regions of Spt16 (S∆C), Pob3 (P∆C), or both, with or without Nhp6, stained with Coomassie blue. The arrow indicates the region excised to test for Nhp6. d Bands (as in (c)) containing apparent FACT:Nhp6 complexes were excised, subjected to denaturing SDS-PAGE and silver stained. The region of the gel containing Nhp6 protein is shown. Nhp6* shows Nhp6 level in the empty area of the gel from the lane containing Nhp6 only, indicating the background level of Nhp6 detection.
