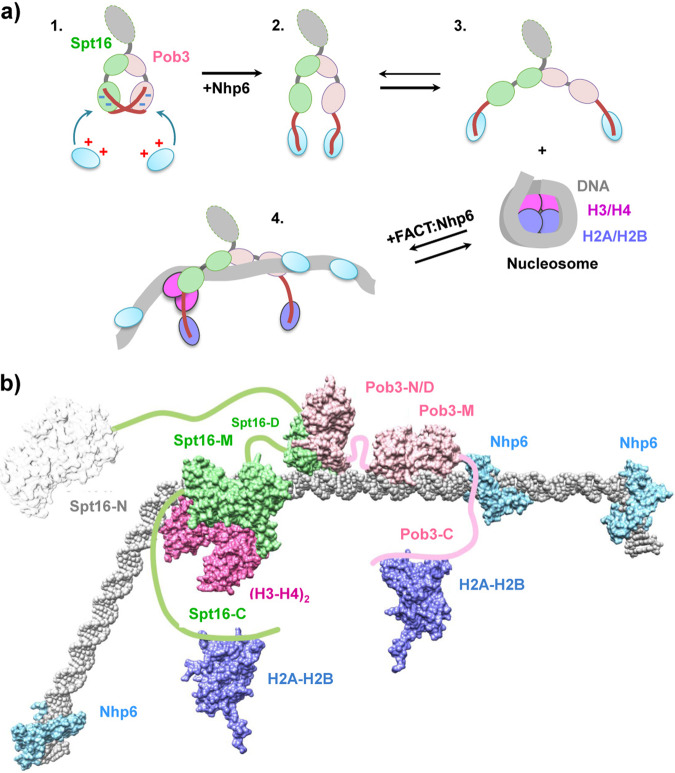
Fig. 5. Model of nucleosome unfolding by FACT.
a Spt16/Pob3 is a mixture of open and closed conformations of the complex (intermediates 1, 2, and 3). Nhp6 interacts with C-terminal domains (CTDs) of Spt16 and Pob3 subunits and induces unfolding of FACT, facilitating FACT-nucleosome complex formation (intermediates 3 and 4). During nucleosome unfolding Nhp6 proteins are transferred from the CTDs to nucleosomal DNA; the vacant CTDs bind to H2A/H2B dimers that become displaced from the DNA. As a result, FACT unfolds the nucleosome in an extended, highly flexible structure (intermediate 4). Other designations as in Fig. 2b. b The proposed structure of the unfolded FACT:Nhp6:nucleosome complex. The (H3-H4)2:Spt16-M:DNA complex is based on the molecular modeling described in Supplementary Fig. 9b, with putative positions of other components inserted using Chimera52 and the published structures of Spt16-N (3BIQ), Pob3-N/D:Spt16-D (4KHB), Pob3-M (2GCL), H2A/H2B (1ID3), and Nhp6:DNA (1J5N), with connectivity based on the locations of the inherently unstructured regions of each protein.