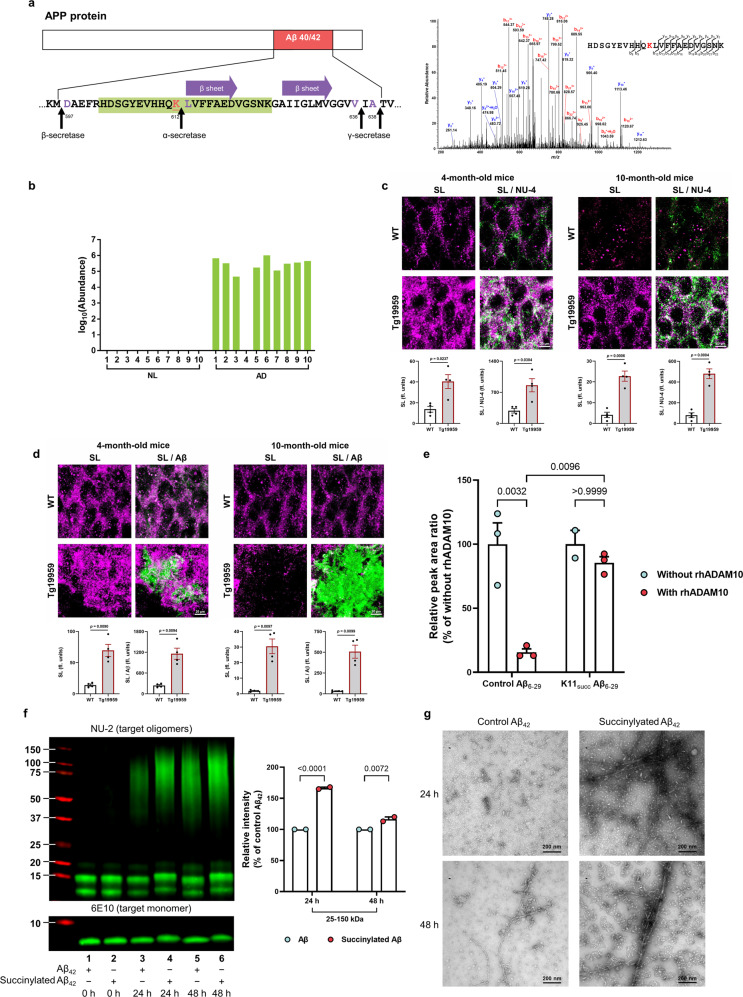
Fig. 5. Succinylation occurs uniquely on APP from AD patients, in early stages of plaque formation in mouse models and disrupts APP processing.
a Location and identity of succinylation K612 near the Aβ region. Residues are numbered according to APP695 sequence. Purple amino acids refer to α- or β- or γ- cleavage sites. The red underlined lysine refers to succinylated K612. Purple arrow represents the two central strands of the β-sheet (Leu613-Asp619 and Ala626-Val632). Green highlights the peptide identified in the MS. MS2 spectrum of m/z 686.57444+ leads to confident identification of a succinylated peptide from APP protein with K612 succinylation site being highlighted in red text. b Abundance of succinylation K612 found in brains from 10 controls and 10 AD patients. Data transformed by log10 (abundance) for normalization purposes and to facilitate presentation. c Confocal microscope analysis of the colocalization of succinylation (magenta) and amyloid oligomers (green) in the hippocampal region of 4 and 10-month-old Tg19959 or wild-type (WT) mice (n = 4 per each group, two-tailed Student’s t-tests). d Brain sections were stained against Aβ plaques (green) and succinyl lysine (magenta). Quantitative analysis of the colocalization of succinylation and plaque pathology in the hippocampus of 4 and 10-month-old Tg19959 or WT mice (n = 4 per each group, two-tailed Student’s t-tests). e Succinylation blocks α-cleavage. Peptides were incubated for 24 h with or without rhADAM10. Peak area ratio values were calculated and are shown relative to corresponding controls without rhADAM10. Each sample was run in triplicate and data were expressed as the mean with SEM (n = 3 biologically independent samples, two-way ANOVA followed by Bonferroni’s multiple comparisons test; except for one sample from the group of succinylated peptide without rhADAM10 was damaged). f Western blot analysis of succinylated and control Aβ42 from aggregation assay showed that the succinylation generates more oligomerized Aβ even after a long incubation. The data were expressed as the mean with SEM (n = 2 biologically independent samples, two-way ANOVA followed by Bonferroni’s multiple comparisons test). g Two timepoints from aggregation assay were analyzed by negative-staining electron microscopy. This experiment was performed once. Source data are provided as a Source Data file.