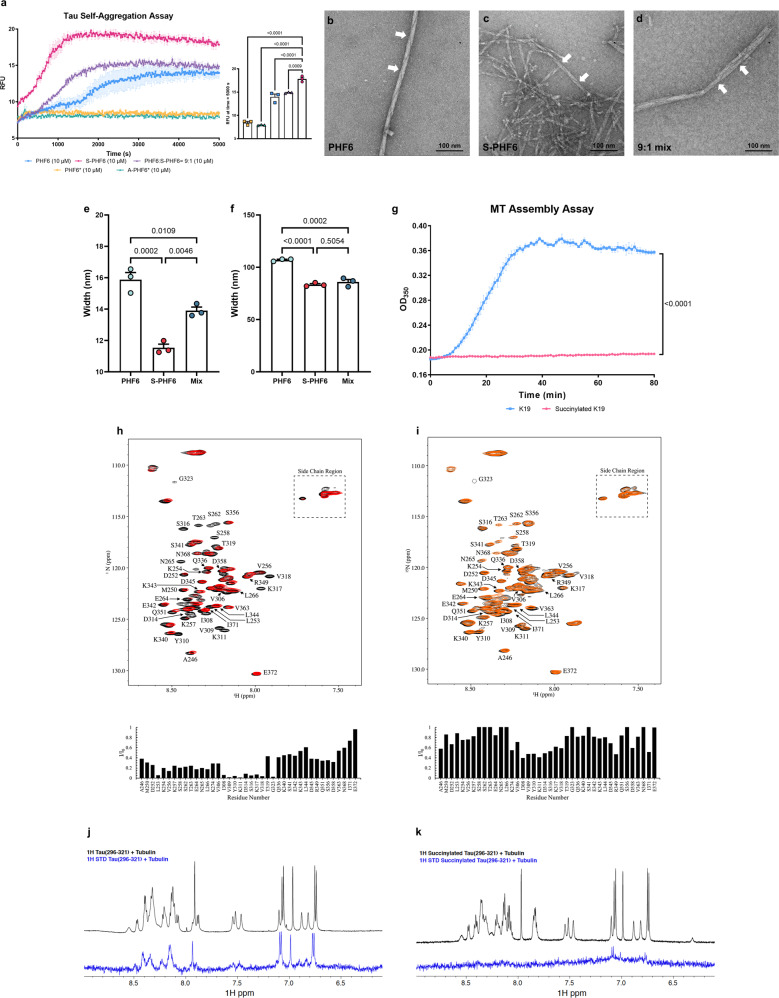
Fig. 7. The succinylation of K311 on tau promotes AD like features in tau pathology.
a Succinylation promotes self-aggregation of tau. Tau peptides (10 μM) were in presence of 2.5 μM heparin: PHF6 (blue squares), S-PHF6 (magenta circles), PHF6:S-PHF6 = 9:1 (purple triangle), PHF6* (yellow square), A-PHF6* (lake green triangle). Error bars represent SEM deviation from the mean. All statistical analysis was implemented at time = 5000 s (n = 3, one-way ANOVA followed by Tukey’s multiple comparisons test). Experiments repeated three times with similar results. b–d Negative stain electron microscopy of in vitro polymerized PHFs after 24 h incubation. b 50 μM PHF6; c 50 μM S-PHF6; d 50 μM mixture (PHF6:S-PHF6 = 9:1). White arrows denote paired helical filaments. N = 3 independent biological replicates. Scale bar is 100 nm. e, f The width and height of the fiber helix found in polymerized PHFs after 24 h incubation in vitro. Error bars represent SEM deviation from the mean (n = 3 different fields per group, one-way ANOVA followed by Tukey’s multiple comparisons test). g Inhibition of assembly reaction of K19 and microtubules by succinylation of K19. Incubations (30 min) were with 30 μM succinylated K19 (magenta circles) or non-succinylated K19 (blue Squares). Error bars represent SEM deviation from the mean. All statistical analysis was implemented at time = 80 min (n = 3, two-way ANOVA followed by Bonferroni’s multiple comparisons test). Experiments repeated three times with similar results. h, i Succinylation of K19 weakens its interactions with T2R.1H,15N HSQC spectra were recorded for unmodified and succinylated K19 in the absence (black) and in the presence (red for unmodified K19, orange for succinylated K19) of T2R. Unmodified or succinylated 15N K19 spectra (assignments for well-resolved residues as indicated) exhibit intensity loss for multiple residues including Ile308, Val309, Tyr310, Lys311 in the presence of T2R. j, k Succinylation of K311 weakens the interactions of tau peptide (296–321) with tubulin. Comparison of 1D 1H spectra (black) and saturation transfer difference NMR spectra (blue) of unmodified tau peptide (296–321) or K311-succinylated tau peptide (296–321) in the presence of 20 μM tubulin. Source data are provided as a Source Data file.